Introduction
Water deficit, without a doubt one of the most critical stress situations, having a significant impact on crop growth and development and thus affecting crop productivity, and as a result, food security, is becoming a major concern around the world. It causes changes in fundamental plant morphophysiology and biochemistry and water loss, which reduces stomatal opening, chlorophyll content, and photosynthesis rate, potentially reducing plant growth and productivity (Xiang et al. 2013, Iqbal et al. 2020). Photosynthesis is a multi-step process that turns light energy into chemical energy, including photosynthetic electron transport and the carbon reduction cycle (Berry et al. 2013). In many plants, water-deficit stress causes stomatal closure, a decrease in transpiration rate and carbon dioxide assimilation capacity, and reduces the activities of photosynthetic carbon reduction cycle enzymes, including 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and phosphoenolpyruvate carboxylase (PEPC) (Chaitanya et al. 2003), as well as the efficiency of photosynthetic electron transport and photosystem II (Xiang et al. 2013). In plants, Rubisco is composed of eight large subunits (LSUs) encoded by chloroplast rbcL gene and eight small subunits (SSUs) encoded by a family of nuclear rbcS genes (Lin et al. 2020).
Water-deficit stress considerably reduces the amount of chlorophyll. Chlorophyll metabolism may significantly affect the assembly of photosynthetic machineries as well as communication between chloroplasts and nuclei (Flexas et al. 2006, Tanaka and Tanaka 2006). The incorporation of Mg2+ into protoporphyrin IX, catalyzed by magnesium protoporphyrin IX-chelatase (Mg-Ch), a three-subunit (ChlI, ChlD, and ChlH) enzyme, is the first unique step in chlorophyll biosynthesis. Chlorophyllase (Chlase), which catalyzes ester bond hydrolysis to produce protochlorophyllide and phytol, is the first enzyme thought to be involved in chlorophyll degradation (Santos 2004). Water- deficit stress-induced increase in Chlase activity and gene expression may result in a loss of chlorophyll accumulation (Banaś et al. 2011). Low Mg-Ch activity was also linked to a lack of chlorophyll. Under magnesium deficiency, reduced expression levels of Mg-Ch and chlorophyll synthase, inhibited chlorophyll synthesis (Zhou et al. 2011).
Silicon (Si) has attracted a lot of attention because it has been shown to improve plant tolerance to a wide range of abiotic stress factors (Coskun et al. 2019). Several studies have shown that Si can increase a plant's ability to withstand drought by either speeding up photosynthetic activity or slowing down transpiration. Si enhances crop growth, production, and quality by affecting photosynthetic activity, nitrogen uptake, and resilience to stress factors (Cooke and Leishman 2011, Liang et al. 2015, Ahanger et al. 2020). It was reported that Si mitigates low phosphorus stress by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis (Zhang et al. 2019). Due to improved water retention, Si reduces drought stress in a variety of plants (Gong and Chen 2012, Liu et al. 2014, Khan et al. 2020, Desoky et al. 2020, Verma et al. 2020).
Rutin (Rut), a flavonoid phenolic compound found in plants such as asparagus (Wang et al. 2003), has antioxidant properties and has been shown to reduce lipid peroxidation (Yang et al. 2008). In comparison to other antioxidant compounds, little research has been conducted to determine the effects of rutin on stressed plants (Ferdinando et al. 2012, Ismail et al. 2015, Singh et al. 2017). In recent studies, it was revealed that rutin improved salt stress tolerance in maize seedlings by modulating osmolyte accumulation and antioxidant capacity (Sezgin Muslu 2024).
The roles of exogenous rutin in protecting plants from abiotic stress factors remain to be fully determined. Moreover, reports showing the effects of rutin and silicon application alone and/or in combination on osmotic stress are limited. In a previous study, the combined application of rutin and silicon alleviated osmotic stress in maize seedlings by triggering the accumulation of osmolytes and antioxidants’ defense mechanisms (Altansambar et al. 2024). However, no attempt has been undertaken to determine how combined application of silicon and rutin affects photosynthesis in plants subjected to osmotic stress. There is also insufficient evidence to explain the effects of rutin and silicon applications on the activities and gene expressions of some key enzymes involved in chlorophyll metabolism and photosynthetic processes. Therefore, in the current study, it was hypothesized that (1) Rut and Si might sustain maize seedlings’ osmotic stress tolerance by improving photosynthetic capacity and chlorophyll metabolism, and (2) Rut might play an important role in the prominent effects of combined applications of Rut and Si in relieving osmotic stress. Our study will provide new information on the changes in photosynthetic capacity and chlorophyll metabolism of Rut and Si at the biochemical and molecular level.
Plant material, experimental conditions and treatments
Zea mays L. seedlings were grown hydroponically in Hoagland’s solution (Hoagland and Arnon 1950) in a growth chamber (day/night temperatures of 25/22 °C, 60 ± 2% relative humidity, and photosynthetic photon flux density of 400 µmol m-2 s-1 with a 16-h photoperiod). After 21 days of growth, rutin (Rut, 60 mg L-1) and silicon (Si, 1 mM) were applied to the seedlings. In our previous study, with concentrations of rutin (30, 60, and 90 mg L-1) and silicon (0,5, 1, and 2 mM), plants under osmotic stress (10% and 15% (w/v) polyethylene glycol) pretreated with 60 mg L-1 Rut and 1 mM Si were found to better maintain water status and had lower membrane damage (Altansambar et al. 2024). Si treatment was added using calcium silicate (CaSiO3) (Sigma Aldrich, USA). For Rut treatment, rutin hydrate was obtained from Sigma Aldrich, USA. After 24 h, seedlings were exposed to osmotic stress induced by adding 10 and 15% (w/v) polyethylene glycol (PEG6000) to Hoagland’s solution for 48 h. Seedlings treated with Hoagland’s solution without PEG were used as the control group. Therefore, we designed nine different treatments: (1) mock; (2) moderate stress: 10% PEG; (3) severe stress: 15% PEG; (4) Rut application before moderate osmotic stress (Rut + 10% PEG); (5) Si application before moderate osmotic stress (Si + 10% PEG), (6) Rut and Si application before moderate osmotic stress (Rut + Si + 10% PEG); (7) Rut application before severe osmotic stress (Rut + 15% PEG); (8) Si application before severe osmotic stress (Si + 15% PEG); (9) Rut and Si application before severe osmotic stress (Rut + Si + 15% PEG). The experimental plan was arranged in a completely randomized design with five replicates, providing a total of five containers with a total of five plants per treatment. After treatments, 24-day-old seedlings were harvested and subjected to biochemical and molecular analysis.
Determination of total chlorophyll content
The total chlorophyll content was determined using Arnon's method (1949). At 0-4 °C, fresh leaf samples (0.1 g) were extracted with 80% acetone. The extracts were centrifuged for 10 min at 15000 g. A spectrophotometer was used to measure absorbance of the supernatant at 645 and 663 nm. The amount of total chlorophyll (mg chlorophyll per fresh tissue) was calculated using the following equation:
mg chlorophyll g-1FW = (20.2 × (A645) + 8.02× (A663) ×
where, A is the absorbance at specific wavelengths; V is the final volume of chlorophyll extract in 80% acetone and W is the fresh weight.
Determination of gas exchange parameters
The LI-6800 Portable Photosynthesis System (LI-COR Biosciences, Inc., Lincoln, NE, USA) was used to quantify the net photosynthetic rate (Pn), transpiration (E), stomatal conductance (gsw), and intercellular CO2 concentration (Ci) of Zea mays. Five plants were included in each group, and ten measurements were taken manually from the upper most fully developed third leaf of each plant at 5-second intervals. The following circumstances were used for the measurements: light intensity of 250 µmol m−2 s −1 (Demiralay 2022), block and leaf temperatures of 25 °C, and relative humidity of 60%. The integrated CO2 mixer in the portable photosynthetic system allowed for independent adjustment of the CO2 concentration. After the leaf was clamped, it was held for at least 30 min until the values of reference CO2 and sample CO2 reached equilibrium at 400 µmol mol–1 CO2.
Determination of chlorophyll fluorescence parameters
A Multi-Mode Chlorophyll Fluorometer (OS5p, Opti-Sciences, Inc., Hudson, NJ, USA) was used to measure chlorophyll fluorescence (CF). Three seedlings were chosen at random for each group and used to measure CF parameters. The leaves were maintained in the dark for 20 min before the Chl fluorescence was measured. A modest red light (0.1 µmol m−2 s −1) was used to determine the minimal fluorescence (F0) after 20 min of acclimation to darkness using the dark leaf clip. The maximum fluorescence (Fm) was then measured using an 8 sec saturating pulse (8000 µmol m−2 s−1) (Nar et al. 2009). Then, in plants exposed to actinic light (500 μmol m–2 s–1) (Chen et al. 2019) Fm', the maximum fluorescence observed in a light-adapted state when all PSII reaction centers are closed and Fs, the fluorescence level measured under steady-state photosynthesis conditions (Krause and Weis 1991), were also determined. The fluorescence parameters were calculated using the following formulas (van Kooten and Snel 1990): the maximum quantum yield of PSII photochemistry, Fv/Fm = (Fm - F0)/Fm; photochemical quenching of variable chlorophyll fluorescence, qP = (F'm -Fs)/(F'm-F'0); and nonphotochemical chlorophyll fluorescence quenching, NPQ = (Fm-F'm)/Fm. The effective quantum yield of ФPSII = (Fm'-Fs)/Fm') of Genty et al. (1989), and electron transfer rate (ETR) of Nar et al. (2009) were also determined.
Determination of Rubisco activity
Extraction buffer (50 mM Tris-HCl, 0.1% (w/v) mercaptoethanol, 12% (v/v) glycerol, 10 mM magnesium chloride (MgCl2), 1 mM EDTA, and 1% (w/v) polyvinylpolypyrrolidone (PVPP-40) was used to prepare extracts from the samples (Parry et al. 1997). The protein contents of the extracts were measured as described by Bradford (1976), with bovine serum albumin as a standard. Rubisco activity was measured according to Sawada et al. (2003) method. The activity was measured at 25°C for 5 min by adding 100 µl of supernatant into 900 µl of assay buffer containing 50 mM of HEPES-KOH (pH 8.0), 1 mM of EDTA, 20 mM of MgCl2, 25 mM of dithioerythritol, 10 mM of NaHCO3, 5 mM of ATP, 0.15 mM of nicotinamide adenine dinucleotide (NADH), 5 mM of creatine phosphate, 0.6 mM of ribulose-1,5-bisphosphate (RuBP), 10 units of phosphocreatine kinase, 10 units of glyceraldehyde-3-phosphate dehydrogenase and 10 units of phosphoglycerate kinase. The enzymatic activity was determined via the decrease in absorbance at 340 nm using extinction coefficient of 6.22 mM−1 cm−1. Total Rubisco activity was expressed in units per milligram of proteins.
Determination of chlorophyllase activity
To determine the activity of chlorophyllase (Chlase), extraction was performed by a slight modification of the method described by Yang et al. (2004). The leaf samples (0.1 g) were homogenized with extraction buffer (5 mM potassium phosphate buffer (pH 7), 50 mM KCl, and 0.24% Triton X-100). After extraction, to remove chlorophyll, the samples were treated with cold acetone, followed by incubation at 30 °C for 30 min in the dark. The samples were then centrifuged at 15000 g for 15 min. Chlase activity was determined by adapting the method of McFeeters et al. (1971). A standard reaction mixture was prepared containing the reaction buffer (100 mM sodium phosphate buffer (pH 7), 0.24% Triton X-100) (2 mL), 1 μmol mL-1 chlorophyll a (0.2 mL) as a substrate and supernatant (0.3 mL), and the reaction was stopped using 0.5 mL of 10 mM KOH with incubation at 30 °C for 30 min. After that, 5 mL of hexane/acetone (3:2, v/v) was added to 1 mL of reaction medium. The content of chlorophyllide a in the acetone phase was determined spectrophotometrically at 667 nm using extinction coefficient of 74.9 mM−1 cm−1. Chlase activity was expressed as production of chlorophyllide a. Proteins were determined by the method of Bradford (1976). The activity was expressed in units per milligram of proteins.
Determination of the expression levels of the rbcL, rbcS, Mg-ChlI, and Chlase genes
Fresh samples (0.1 g) were used for total RNA isolation. After the samples were broken down in a tissue homogenizer, total RNA isolation was performed using a total RNA isolation kit (Favorgen FavorPrep Plant Total RNA Mini Kit) following the kit's protocol. A NanoDrop spectrophotometer was used to determine the quantity and purity of the RNA samples. The RNA samples were assessed for purity before being stored at -80 °C for cDNA extraction. From the isolated total RNA samples, 2000 ng of cDNA was obtained per group using the High Capacity cDNA Reverse Transcription Kit from Applied Biosystems USA. The synthesized cDNAs were stored at -20 °C until real time PCR analyses were performed.
The resulting cDNAs were used to identify gene expression using real-time PCR. 5 HOT FIREPol EvaGreen qPCR Supermix and the CFX Connect Real-Time PCR System were used for analysis. The real-time PCR protocol was modified from the Solis BioDyne instructions: 12 min at 95 °C, 45 cycles of 15 sec at 95 °C, 30 sec at 60 °C, 30 sec at 72 °C, and 0.5 °C increments from 65 °C to 95 °C for the melt curve. Each biological repeat was examined as three technical replications, with the average technical error accepted as 0.5 (± 1) Cq values. Furthermore, gene-specific primers were used to investigate the levels of expression of the genes (Tab. 1). The data obtained as a result of the analysis were normalized to the β-actin reference gene and presented as relative gene expression using the 2-ΔΔCT method, following the protocol outlined by Bookout and Mangelsdorf (2003).
Tab. 1. The sequences of specific primers of genes, used for qRT-PCR analysis. Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL), ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (rbcS), magnesium chelatase subunit I (Mg-ChlI), chlorophyllase (Chlase).
Statistical analysis
All experiments were performed five times with five biological replicates in total. The Shapiro-Wilk normality test was used to test the normal distribution of variables. A two-way ANOVA and Tukey's multiple range test was performed at the 0.05 (5%) level using IBM SPSS 23.0 Statistics Package (SPPS Corp., Chicago, IL, USA).
Total chlorophyll content
According to the results of two-way ANOVA (Tab. 2), the total chlorophyll content was significantly (P < 0.001) affected by osmotic stress conditions (OS) and treatments with Rut, Si or both (T). Moreover, the interaction between OS and T factors was also highly significant for the total chlorophyll content, suggesting that the effect of one factor depends on the level of the other factor.
Tab. 2. Results of two-way ANOVA (P-Values, F ratios) for the independent ‘osmotic stress conditions’ (OS), ‘treatment’ (T) and ‘osmotic stress × treatment’ interactions. Pn − net photosynthesis, E − transpiration, gsw − stomatal conductance, Ci − intercellular CO2 concentration, maximum quantum yield of PSII photochemistry − Fv/Fm, the effective quantum yield of PSII − ФPSII, qP − photochemical quenching, NPQ − non-photochemical quenching, rbcL − Rubisco large subunit, rbcS − Rubisco small subunit, Mg-ChlI − magnesium chelatase subunit I, Chlase − chlorophyllase. * indicate significant difference at P < 0.05; ** indicate significant difference at P < 0.01; *** indicate significant difference at P < 0.001.
As shown in Fig. 1, the differences in the concentration of the osmotic stress (10 or 15% PEG) had a significant (P < 0.05) effect on the total chlorophyll content of maize leaves. The stressed seedlings showed 47.25 and 162.02% lower total chlorophyll levels, respectively, in comparison with the control group. However, the total chlorophyll content was significantly enhanced (P < 0.05) by the application of Rut or Si alone and in combination under both osmotic stress treatments, especially moderate stress. Additionally, compared to Rut or Si alone, the combination of Rut and Si was found to further increase the total chlorophyll content (Fig. 1).
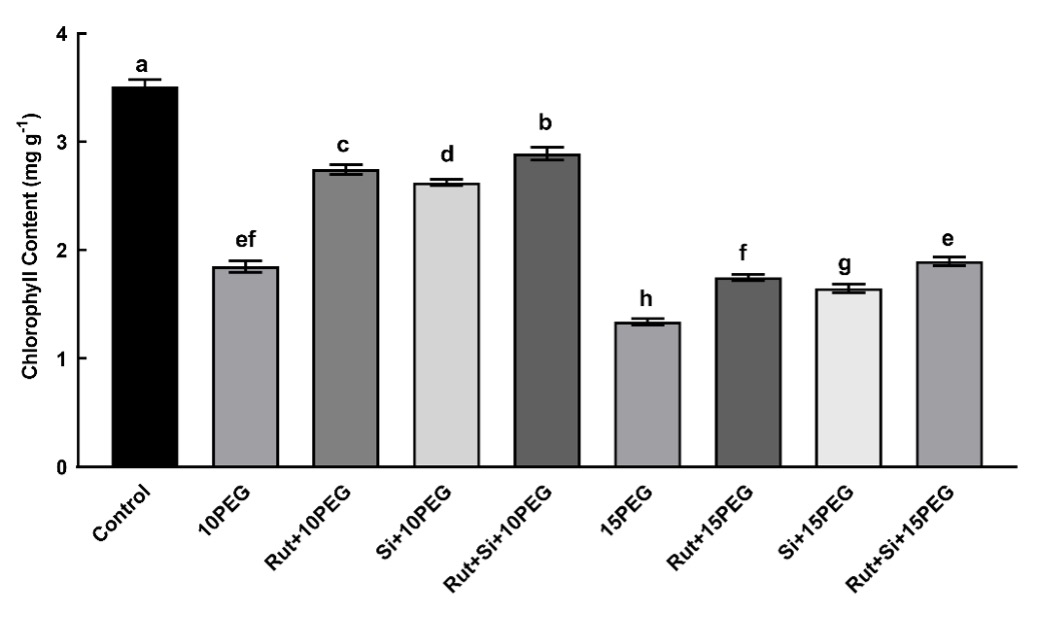
Fig. 1. Effect of the treatments (T) with rutin (Rut), silicon (Si), and their combination (Rut+Si) on total chlorophyll content of maize seedlings under osmotic stress (OS) conditions (10% or 15% polyethylene glycol - PEG). Vertical bars represent standard deviations of the means, N = 5. Data were subjected to two-way ANOVA while Tukey's multiple range test was used for determinating the differences among means for interaction OS × T. Values marked with the different letters denote significant difference (P < 0.05).
Gas exchange parameters
According to results of two-way ANOVA (Tab. 2), both OS and T had highly significant effects (P < 0.001) on net photosynthesis (Pn) and the transpiration rate (E). Similarly, OS and T both significantly affected (P < 0.001) stomatal conductance (gsw) and intercellular CO2 concentration (Ci). The interaction between OS × T was also significant for Pn, E, gsw and Ci, though the F-value for the interaction was small compared to the OS and T factors, indicating a less strong but still significant interaction (P ≤ 0.001).
The Pn, E, gsw and Ci values were significantly decreased (P < 0.05) with 10% and 15% PEG treatments compared to the non-stress group. The decrease in these values was highest in the 15% PEG treated group. However, exogenous Rut, Si and Rut+Si treatments significantly increased (P < 0.05) Pn, E, gsw, and Ci under both osmotic stress conditions. However, the highest values of Pn and E were observed in Rut, Si and Rut+Si treatments under 10% PEG. Furthermore, the highest value of gsw was observed in Si and Rut+Si treatments and the highest value of Ci were detected in Rut+Si treatment under 10% PEG, which were significantly (P < 0.05) different from 15% PEG conditions (Tab. 3).
Tab. 3. Effect of the treatments (T) with rutin (Rut), silicon (Si), and their combination (Rut+Si) on net photosynthesis (Pn), transpiration (E), stomatal conductance (gs) and intercellular CO2 concentration (Ci) of maize seedlings under osmotic stress (OS) conditions (10% or 15% polyethylene glycol - PEG). All values are presented as means ± standard deviation, N = 5. Data were subjected to a two-way ANOVA and Tukey's multiple range test was used to determine the differences among means for interaction OS × T. Values marked with the different letters denote significant difference (P < 0.05).
Chlorophyll fluorescence parameters
Two-way ANOVA analysis (Tab. 2) showed that the content of all chlorophyll fluorescence parameters (Fv/Fm, ФPSII, ETR, qP, and NPQ) was significantly different in respect of osmotic stress conditions (OS), treatments (T) and their interactions (OS × T).
As shown in Tab. 4, the values of Fv/Fm, ФPSII, ETR, qP were significantly (P < 0.05) reduced under both 10% and 15% PEG treatments compared to the non-stressed group.
Tab. 4. Effect of the treatments (T) with rutin (Rut), silicon (Si), and their combination (Rut+Si) on maximum efficiency of PSII (Fv/Fm), the effective quantum yield of PSII (ФPSII), photochemical quenching (qP), non-photochemical quenching (NPQ) of maize seedlings under osmotic stress conditions (OS) (10% or 15% polyethylene glycol - PEG). All values are presented as means ± standard deviation, N =5. Data were subjected to a two-way ANOVA and Tukey's multiple range test was used to determine the differences among means for interaction OS × T. Values marked with the different letters denote significant difference (P < 0.05).
In contrast, values of NPQ were significantly (P < 0.05) increased under both osmotic stresses compared to the non-stress group. Application of Rut or Si, and especially their combination significantly increased values of Fv/Fm, ФPSII and qP under both osmotic stresses, although values were still not as high as in control. In contrast, NPQ values were lower after application of Rut or Si, and especially of a combination of the two, than under osmotic stress alone.
Rubisco activity
According to results of two-way ANOVA (Tab. 2) the Rubisco activity was significantly affected (P < 0.001) by OS and T as well as their interaction (OS × T) indicating strong effects.
Compared to non-stressed plants, osmotic stress significantly reduced the activity of Rubisco, with severe stress (15% PEG) having a more prominent effect. Exogenous applications of Rut, Si, and Rut+Si significantly (P < 0.05) increased Rubisco activity under both osmotic stress conditions, especially moderate stress (10% PEG), compared to the same level of stress alone. Moreover, compared to Rut or Si alone, the combination of Rut and Si was found to further increase Rubisco activity (Fig. 2).
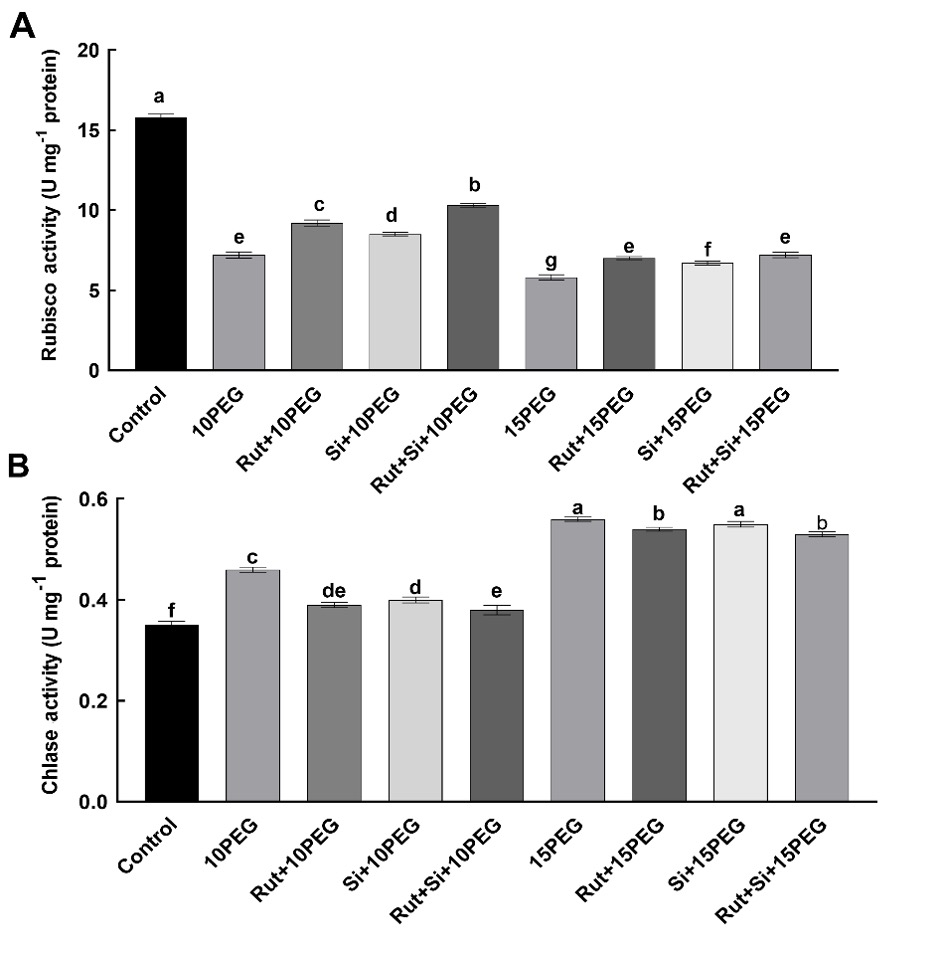
Fig. 2. Effect of the treatments (T) with rutin (Rut), silicon (Si), and their combination (Rut+Si) on Rubisco activity (A) and chlorophyllase (Chlase) activity (B) of maize seedlings under osmotic stress (OS) conditions (10% or 15% polyethylene glycol - PEG). Vertical bars represent standard deviations of the means, N = 5. Data were subjected to a two-way ANOVA and Tukey's multiple range test was used to determine the differences among means for interaction OS × T. Values marked with the different letters denote significant differences (P < 0.05).
Chlorophyllase activity
Two-way ANOVA analysis showed that the chlorophyllase activity was significantly affected by OS and T factors as well as OS × T (P < 0.001) indicating strong effects (Tab. 2).
Both levels of osmotic stress, but especially severe stress (15% PEG) significantly (P < 0.05) increased chlorophyllase activity, compared to the control. Exogenous treatments with Rut and Si decreased the chlorophyllase activity under osmotic stress, compared to the seedlings exposed to the same level of osmotic stress alone but more efficiently at moderate stress. Also, compared to Si alone, seedlings treated with Rut+Si exhibited an even more significant (P < 0.05) decrease in chlorophyllase activity under stress conditions (Fig. 2).
The expression levels of rbcL, rbcS, Mg-ChlI and Chlase genes
According to results of two-way ANOVA, the expression levels of the rbcL and rbcS, endcoding large and small Rubisco subunits, respectively, were significantly different (P < 0.001) in respect of osmotic stress conditions (OS), treatments (T) and their interactions (OS × T) (Tab. 2). As compared to the control, the expression levels of rbcL and rbcS were significantly down-regulated by 10 and 15% PEG. Exogenous applications of Rut, Si, and especially Rut+Si significantly up-regulated the expression levels of rbcL and rbcS under moderate and severe osmotic stress as compared to the same level of stress alone (Fig. 3).
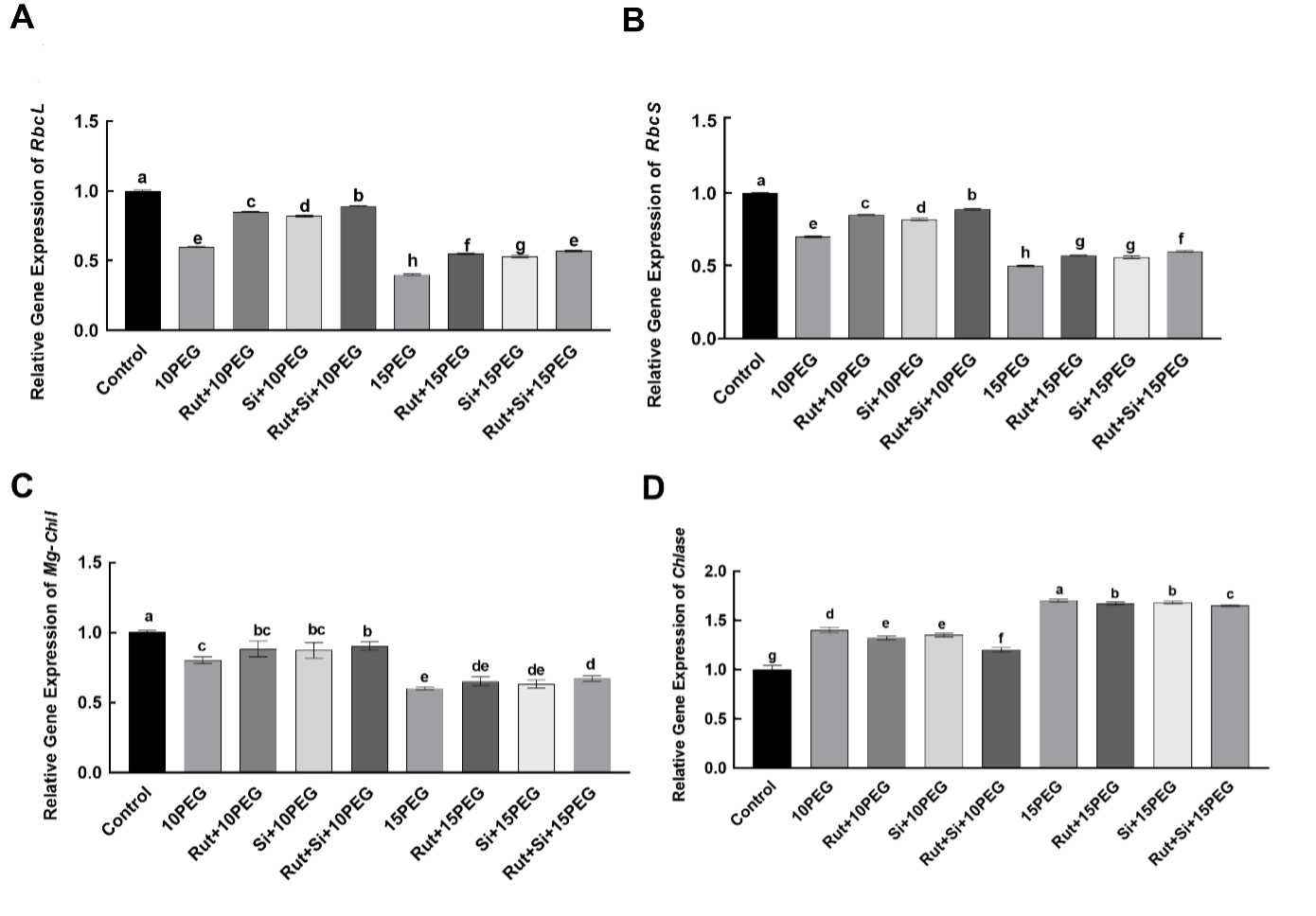
Fig. 3 Effect of the treatments (T) with rutin (Rut), silicon (Si), and their combination (Rut+Si) on the expression levels of of the rbcL (A) and rbcS (B), Mg-ChlI (C) and Chlase (D) genes of maize seedlings under osmotic stress (OS) conditions (10% or 15% polyethylene glycol - PEG). Vertical bars represent standard deviations of the means, N = 5. Data were subjected to two-way ANOVA and Tukey's multiple range test was used for determinating the differences among means for interaction OS × T. Values marked with the different letters denote significant differences (P < 0.05).
Two-way ANOVA analysis showed that the effect of OS factor on the expression levels of the Mg-ChlI was significant with P < 0.001 as well as the effects of T with P = 0.003 (Tab. 2). The expression levels of the Mg-ChlI in maize seedlings treated with 10 and 15% PEG were significantly lower than in the control. In addition, under moderate and severe osmotic stress conditions, the expression levels of Mg-ChlI in seedlings pretreated with Rut, Si, and Rut+Si were higher than under moderate and severe osmotic stress (Fig. 3).
According to results of two-way ANOVA, the expression level Chlase was significantly different (P < 0.001) in respect of osmotic stress conditions (OS), treatments (T) and their interactions (OS × T) (Tab. 2). Both levels of osmotic stress caused a significant increase in the expression level of Chlase, compared to the control. Under moderate and severe osmotic stress, exogenous application of Rut, Si, and especially Rut+Si significantly down-regulated the expression level of Chlase (Fig. 3).
Discussion
Chlorophyll is required to convert light energy to chemical energy, and its depletion limits the photosynthetic process in plants (Kalaji et al. 2017). In current study, two-way ANOVA analysis of variance revealed that both independent variables (OS and T) had significant effects on the total chlorophyll content. Total chlorophyll content decreased in seedlings exposed to osmotic stress, while Rut, Si, and especially Rut+Si applications reduced the negative effects of osmotic stress on chlorophyll content. Consistently with our findings, Si- treated wheat and Eruca sativa L. plants showed a significant increase in chlorophyll content (Maghsoudi et al. 2015, Bukhari et al. 2021). Moreover, Si application improved the chlorophyll content in Fagopyrum esculentum M. plants under aluminium stress (Dar et al. 2022). In another study, total content of chlorophyll in Oryza sativa L., leaves increased during treatment with rutin (Singh et al. 2017). The chlorophyll content could decline due to increased Chlase activity under abiotic stressors (Dawood et al. 2014). Increased Chlase activity has been correlated with chlorophyll degradation in plants under osmotic stress conditions (Altuntaş et al. 2020), which is agreement with our results in maize seedlings where osmotic stress conditions induced significant enhancement of Chlase activity. However, Chlase activity significantly decreased in seedlings pretreated with Rut, Si, and especially Rut+Si under osmotic stress thus contributing to the higher content of chlorophyll content observed. In mustard seedlings exposed to salinity and drought stress, it was found that Chlase activity and chlorophyll degradation were decreased by Si (Alamri et al. 2020). Moreover, we also observed a correlation of Chlase activity with gene expression of Chlase i.e. Chlase activity decreased and the Chlase gene expression was considerably down-regulated in seeedlings under osmotic stress pretreated with Rut, Si, and Rut+Si. Therefore, we can hypothesize that Rut, Si and Rut+Si may have adjusted the transcript levels of genes encoding the Chlase that degrades chlorophyll, which can prevent the bleaching of chlorophyll and preserve photosynthetic activity. In the production of chlorophyll, a crucial regulation and enzymatic step is catalyzed by the heterotrimeric enzyme complex known as Mg-Chl (Rissler et al. 2002). Overexpression of the Mg-chelator H subunit in guard cells has been shown to increase drought tolerance in Arabidopsis thaliana (Tsuzuki et al. 2013). Another study also showed that Si application upregulated the expression of genes encoding enzymes in chlorophyll synthesis, Mg-Chl and chlorophyll oxygenase in cucumber seedlings under excess nitrate stress. In our study, the expression of the Mg-ChlI gene was significantly upregulated in Rut, Si, and Rut+Si applied maize seedlings under osmotic stress. As a result, we can conclude that increased Mg-ChlI gene expression and decreased Chlase expression can confer stress tolerance to maize seedlings.
Damage to photosynthesis can reduce chlorophyll content, causing the chloroplast bilayer membrane to rupture and disrupting coordination between the two photosystems, ultimately decreasing the photosynthesis rate (Lawlor and Cornic 2002, Chaves and Oliveira 2004). Lower Pn could be due to the fact that osmotic stress can lead to a decrease in gsw, Ci, and E as a result of stomatal closure (Chaves et al. 2003). Similarly, in our study, osmotic stress conditions (10% and 15% PEG) significantly decreased values of gas exchange parameters. The decreases were found to be higher at 15% PEG compared to 10% PEG application because severe drought stress can lead to structural and biochemical impairments in light-dependent reactions and carboxylation processes (Ghotbi-Ravandi et al. 2014). Many researchers have reported that exogenous applications of Si can enhance photosynthesis performance under osmotic stress (Maghsoudi et al. 2016, Li et al. 2018, Li et al. 2022, Mavondo-She et al. 2024). Like those studies, our results showed that Rut, Si and Rut+Si significantly increased all of the gas exchange parameters in maize seedlings. The results suggest that exogenous treatments of Rut, Si and Rut+Si can mitigate the adverse effects of osmotic stress on gas exchange due to more efficient light use and improved chlorophyll metabolism through the regulation of the Mg-ChlI and Chlase gene. Hence, those applications can maintain higher chlorophyll content, preserve the photosynthetic machinery and enhance overall photosynthetic performance.
Osmotic stress can have a significant impact on plant photosynthesis resulting in changed chlorophyll fluorescence (Chen et al. 2021). However, exogenous applications of various compounds have been shown to mitigate the negative effects of osmotic stress and enhance chlorophyll fluorescence parameters due to improved photosynthetic performance and overall plant resilience (Hayat and Ahmad 2007, Ashraf and Foolad 2007, Ahmed et al. 2019, Hussain et al. 2023). Many researchers have reported that chlorophyll fluorescence parameters are valuable for assessing PSII activity and functioning of the photosynthetic apparatus in plants under osmotic stress. The maximum quantum efficiency of PSII is linked to photosynthetic efficiency in leaves (Baker and Rosenqvist 2004, Baker 2008) and the Fv/Fm ratio serves as an indicator of photoinhibition or stress damage. In the present study, 10% and 15% PEG reduced Fv/Fm, ΦPSII, and qP values, indicating a potential decline in photosynthetic activity. The reduction in Fv/Fm is likely linked to decreased activity of PSII reaction centers and/or decreased energy transfer efficiency within reaction centers as well as reduced gsw and CO2 availability, suggesting photoinhibition. An increase in NPQ indicating enhanced energy dissipation, as a mechanism to avoid photodamage, observed in stressed plants confirms these results. Additionally, the reductions in ΦPSII and qP may be associated with the changes in Fv/Fm (Maxwell and Johnson 2000). These declines are probably due to altered chlorophyll content, leading to the conclusion that osmotic stress inhibits PSII activity as previously established for maize (Altuntaş et al. 2020). However, applications of Rut, Si, and the combination of Rut+Si mitigated the impairment of photosynthetic parameters, as evidenced by enhanced electron transport under osmotic stress, and improved PSII efficiency.
Abiotic stressors can also reduce photosynthesis by reducing the activity of Rubisco which is the enzyme that fixes CO2 and catalyzes photo-respiratory carbon oxidation (Abdulbaki et al. 2022). We determined that osmotic stress in maize seedlings negatively affected the activity of Rubisco while application of Rut, Si, and Rut+Si alleviated this negative effect. Especially effective was the combination of Rut and Si under moderate osmotic stress. Similarly, Si application increased Rubisco activity in cucumber seedlings under cinnamic-acid-induced stress (Lyu et al. 2022). Rubisco biosynthesis requires a high number of chaperones and involves eight large (Rubisco LSU) and eight small subunits (Rubisco SSU) (Lin et al. 2020). The abundances of transcripts of rbcL encoding LSU and rbcS encoding SSU could enhance photosynthetic properties, photosynthetic efficiency or capacity (Chen et al. 2015). In the current study, it was found that the expression levels of rbcL and rbcS were down-regulated in the maize seedlings under osmotic stress conditions which correlates with decreased activity of Rubisco. Rut, Si, and Rut+Si applications significantly upregulated the expression of rbcL and rbcS genes in seedlings exposed to osmotic stress, consequently also increasing Rubisco activity. Consistently with our study, Si application significantly upregulated the expression of genes encoding Rubisco subunits in tomato seedlings under low-calcium stress (Li et al. 2022). No study has been found in the literature on the way in which Rut application affects the activities of photosynthetic enzymes and the expression of genes encoding these enzymes. In our study, it was revealed that the alleviating effect of Si was further stimulated by Rut. We found that increased expression of genes involved in the regulation of the activity of major photosynthetic enzymes and genes encoding Rubisco following combined application of Rut+Si could be one of the primary causes of enhanced photosynthesis under osmotic stress, suggesting that the synergistic effect of Rut and Si may positively modulate CO2 assimilation.
In conclusion, the results of this study show that the application of Rut, Si, or Rut+Si to maize plants under osmotic stress can suppress chlorophyll degradation and stimulate chlorophyll synthesis and Rubisco activity, thereby preserving photosynthetic activity. Rut, Si, and especially Rut+Si applications have the potential to maintain photosynthesis or alleviate photosynthetic damage in plants under osmotic stress. Our research was aimed at a better understanding of the key mechanisms underlying Rut+Si-mediated osmotic stress tolerance. Furthermore, the study may help to address gaps in knowledge regarding the effects of Rut on stress tolerance. By using the benefits of Rut and Si, researchers may be able to plan more sustainable agriculture. Based on the findings of the current study, we can conclude that the application of Rut and Si or their combination to plants subjected to osmotic stress can improve plant growth and provide the plants with an advantage under stress conditions.
