Introduction
Dairy cattle breeding is one of the most demanding livestock productions, where the farmers have to analyse a large amount of information on a daily basis and know how to react in a timely manner to prevent possible problems in production (Gantner, 2020; Gantner et al., 2021). Furthermore, the farmer must be aware of the genetic and environmental factors of the various traits of interest in milk production in order to be able to optimize management and realize the genetic potential of animals on the farm. An efficient dairy cattle farming implies gravidity and calving on an annual basis, whereby the transition period and the beginning of lactation represent the most stressful period in the production cycle of dairy cows (Gantner, 2020). There are sets of various disorders that could occur during this period due to different factors such as dietary modifications, negative energy balance, lowered feed intake, weight loss, and hypocalcemia (Ametaj, 2017). The most frequent metabolic disorders that occur in dairy cows postpartum and during the first month of lactation are sub-acute and acute ruminal acidosis, laminitis, ketosis, fatty liver, displaced abomasum, milk fever, downer cow, retained placenta, liver abscesses, metritis, mastitis and bloat (Ametaj, 2017). It is also important to emphasise that the occurrence of one metabolic disorder is highly associated with other ones (Suthar et al., 2013; Ametaj, 2017). For instance, cows affected by milk fever are more predisposed to mastitis, retained placenta, metritis, ketosis; while cows affected by acidosis are more prone to laminitis, milk fever, mastitis, and fatty liver (Suthar et al., 2013; Ametaj, 2017).
Subacute and acute ruminal acidosis are highly prevalent disorders in high-producing dairy herds especially in early lactation and in cows with a high intake of dry matter (Ametaj, 2017; Bramley et al., 2005; O’Grady et al., 2008). There is a higher risk of acidosis in early lactation cows because of decreased absorptive capacity of the rumen, inadequately adapted rumen microflora, and the fast introduction of high-concentrate rations (Dirksen et al., 1985). Furthermore, cows that are at peak dry matter intake are at a high risk of acidosis prevalence due to the higher amount of acids created in the rumen (Oetzel, 2005). The symptoms of acute acidosis could be relatively easy to identify allowing the farmer to react quickly and on time; while on the other hand, the symptoms of subclinical acidosis are more difficult for accurate identification (Oetzel, 2005). Therefore, subclinical acidosis could be inaccurately identified or unidentified resulting in high economic losses to dairy farms. The most typical clinical symptom of subclinical acidosis is decreased or varying feed intake, reduced milk production, decreased milk fat content, poor body condition score, high culling rate, diarrhoea, and laminitis (Ametaj, 2017). Acute acidosis in dairy cow is characterised by a significant decrease in ruminal pH (≤5.0), increased concentrations of volatile fatty acids (VFA) and lactate in the rumen as well as a significant reduction in the number of protozoa (Ametaj, 2017). The symptoms of acute acidosis are low appetite and feed intake, diarrhoea, accelerated respiration, salivation, depression, lethargy, lameness, and probably death. Furthermore, low milk fat content in a cow with the disorder is commonly used as an indicator of the occurrence of subclinical acidosis. The most reliable diagnostic test for subclinical acidosis is the measurement of ruminal pH by stomach tubing or rumenocentesis (Enemark, 2008; Ametaj, 2017). Oetzel (2017) emphasised that subacute ruminal acidosis is a frequent problem in lactating dairy cows that induces persistent health problems, reduces feed efficiency, and increases the environmental impact of dairy farm.
Ketosis represents a metabolic disorder that occurs in dairy cattle as a result of negative energy balance (animal’s demands for energy exceed the intake of energy; usually due to increased daily milk production). When large amounts of body fat are used as an energy source to maintain milk production, fat is sometimes mobilised faster than the liver can correctly metabolise it. Consequently, the ketone production exceeds ketone utilisation which results in the occurrence of ketosis. Similar to acidosis, ketosis can also occur in clinical and subclinical forms as well. The prevalence of ketosis varies in relation to the stage of lactation, parity, season and breed. According to Gillund et al. (2001) clinical ketosis most frequently occurs in high yielding cows at the beginning of the lactation (between the 2nd and 7th week of lactation) mostly because of poor feeding and management. Itle et al. (2015) reported the occurrence of subclinical ketosis in a range from 10 % to 60 % and clinical ketosis in a range from 2 % to 15 % during the first month of lactation. Usually, a high yielding dairy cows have negative energy balance throughout early lactation since the energy intake cannot suffice the demands of milk production (Cao et al., 2017; Xu et al., 2015). An extremely negative energy balance usually leads to ketosis, which can result in decreased milk production, fast weight loss and noticeable loss of body condition, less rumination, reduced reproductive performance, anorexia, dry faeces, and increased risk of other related disorders (fatty liver, displaced abomasum, and metritis; Kaufman et al., 2016; Suthar et al., 2013; Walsh et al., 2007). Ketosis implies a high concentration of ketone bodies (acetone, acetoacetate, beta-hydroxybutyrate) in all body fluids. Subclinical ketosis is defined as high serum ketone body concentrations without recognition of clinical signs. Diagnosis of ketosis is based on the appearance of risk factors (animal in early lactation), clinical signs, and elevated ketone body concentrations in blood, urine, or milk (the β-hydroxybutyrate concentrations, BHB >1.0 mmol/L (10.4 mg/dL) in blood or 1.4 mmol/L (14.6 mg/dL) in serum are considered as diagnostic of subclinical ketosis). Since clinical ketosis produces economic losses to dairy farmers through treatment costs, reduced milk production decreased reproduction efficiency and increased involuntary culling (Rajala and Gröhn, 1998; Kaufman et al., 2016; Suthar et al., 2013; Walsh et al., 2007), timely detection and elimination of disorder in subclinical phase is extremely important.
For early detection of metabolic disorders, it is necessary to monitor health status of dairy cows at the herd level. In that case, usage of test-day records (daily milk yield, fat and protein content as well as fat to protein ratio) represents the cost-effective and non-invasive diagnostic method (Duffiled et al., 1997; Eicher, 2004). The optimal values of fat to protein ratio (F/P), depending on research, ranged from 1-1.25 (Gravert, 1991), with upper threshold amounted 1.33 (Duffield et al., 1997), 1.4 when it indicates energy deficit and subclinical ketosis if ketone bodies are present (Haas and Hofirek, 2004), and 1.5 when it indicates the prevalence risk of subclinical ketosis (Richardt, 2004). Eicher (2004) considered F/P and daily milk production as indicators of metabolic disorders prevalence (acidosis, ketosis).
The objective of this research was to determine variability and co-variability of daily production traits (daily milk yield, daily fat and protein content as well as F/P ratio) and biochemical parameters in plasma and milk samples as well as haematological parameters. Therefore, the correlation between the daily production traits and biochemical and haematological parameters were determined. Also, values of biochemical and haematological parameters depending on F/P ratio classes (indicating risk of acidosis, ketosis, or normal status of animal) were determined.
Materials and methods
The study was conducted on a commercial indoor dairy cattle farm located in East Croatia. During a three months period, on monthly basis, blood and milk samples were taken from 25 Holstein cows with an average daily milk yield 39.30±9.02 kg. None of the animals included in the trial didn't suffer from any metabolic or reproductive disorders. Animals were fed on the basis of set norms for dairy cows based on the level of productivity, that is, with total mixed ration (TMR) to which a mixture with 40 % crude protein was added. The proportion of the mixture was dosed depending on animal's productivity. Blood samples of cows were taken from the coccygeal vein into tubes with lithium heparin anticoagulant (Becton Dickinson, Plymouth, England, UK). Samples were centrifuged (1.500 g/10 min at 4 °C) and plasma separated and frozen at -80 °C until analyses. Samples for haematological analyses were taken into Ca-EDTA tubes (Becton Dickinson, Plymouth, England, UK) and analysed within 2 hours on Poch 100Veff (Sysmex, Japan). Samples of milk were taken into clean tubes, centrifuged (12.000 g/30 min at 4 °C) and the obtained milk plasma was separated and stored at -80 °C until analyses. Biochemical parameters in blood and milk plasma were determined using an automatic clinical chemistry analyser Beckman Coulter AU400 (Beckman Coulter, Germany). The concentration of β-hydroxybutyrate (BHB) was determined using commercial kits (Randox Laboratories Ltd, Crumlin, UK) by the enzymatic colorimetric method. Additionally, test-day records (from regular milk recording containing data on daily milk yield and daily fat and protein content) of the cows under the experiment were taken from the central database of HAPIH (Croatian Agency for Agriculture and Food). Test-day records were corrected accordingly to the ICAR guidelines (2017). With regard to the F/P value, records were divided into three classes: F/P ratio <1.1 (acidosis risk); F/P ratio in [1.1, 1.5] (normal status); F/P ratio >1.5 (ketosis risk). Table 1 presents the basic statistical parameters of daily production traits (daily milk yield, daily fat and protein content as well as F/P ratio).
Table 1. Basic variability of daily production traits of Holstein cows
Correlation coefficients were determined between the daily production traits and biochemical parameters in plasma and milk, as well as haematological parameters. Furthermore, the variability of biochemical and haematological parameters due to on F/P ratio classes were tested using least square means in GLM procedure in SAS (SAS Institute Inc., 2019). The following statistical model was used:
yijklm = μ + b1(di / 305) + b2(di / 305)2 + b3 ln(305 / di) + b4 ln2(305 / di) + Pj + Mk + Dl + eijklm
where:
yijklm = estimated biochemical or haematological parameters;
μ = intercept;
b1, b2, b3, b4 = regression coefficients (lactation curve by Ali and Schaeffer, 1987);
di = days in milk (i = 11 to 345 day);
Pj = fixed effect of parity j (j = II, III, IV, V);
Mk = fixed effect of experiment month k (k = May, June, July),
Dl = fixed effect of F/P ratio classes (acidosis risk, normal status, ketosis risk),
eijklm = residual.
Scheffe’s multiple comparisons in PROC GLM (SAS) were used to test the significance (p<0.05) of the differences in biochemical and haematological parameters due to on F/P ratio classes.
Results and discussion
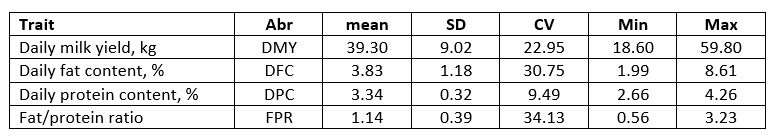
Correlation coefficients between the daily milk production traits and biochemical parameters in plasma ranged between 0.009 (negligible relationship) to 0.400 (strong relationship) (Table 2). A week or negligible negative relationship was determined between the F/P ratio and aspartate amino transferase (AST), γ-glutamil transferase (GGT), glucose (GUK), urea (UREA), β-hydroxybutyrate (BHB) while a week positive relationship existed with protein (PRO) and triglyceride (TGC) in plasma. A moderate negative relationship indicates the decrease of Fe in plasma with an increasing F/P ratio meaning that cows at risk of ketosis will experience a decrease in plasma Fe. A weakened immune system during the post-partum period makes dairy cows more prone to bacterial infection. Restriction of iron availability is one of the anti-microbial defence mechanisms. A common mechanism of hypoferremia of inflammation is a cytokine-driven increase in hepcidin, which downregulates ferroportin and thereby decreases iron flow into the extracellular fluid from all sources (Ganz, 2018). The risk of ketosis prevalence is most pronounced in the post-partum period. The correlation between iron concentration and F/P ratio as a ketosis risk indicator is present but the reason for change comes from different mechanisms.
Table 2. Correlation coefficients between milk traits and biochemical parameters in plasma
*DMY - daily milk yield (kg); DFC - daily fat content (%); DPC - daily protein content (%)
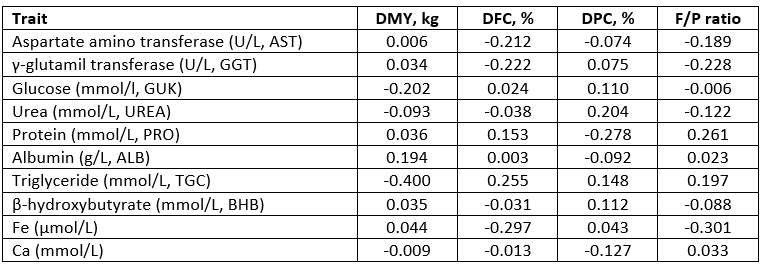
The correlation coefficients between the daily milk production traits and haematological parameters indicate a negligible or week relationship (in a range between 0.003 to 0.200) (Table 3). The negative and weak relationship between the F/P ratio and red blood cells (RBC) indicates a decrease in RBC in the animals when the F/P ratio in milk increases, that is when animals are at risk of ketosis prevalence. Furthermore, a negative relationship was also determined between the F/P ratio and haemoglobin (HGB) and haematocrit (HTC). A determined positive correlation between the F/P ratio and white blood cells (WBC) could be explained by the rise in WBC as a consequence of milk gland infection in the post-partum period (Alhussien et al., 2015).
Table 3. Correlation coefficients between milk traits and haematological parameters
*DMY - daily milk yield (kg); DFC - daily fat content (%); DPC - daily protein content (%)
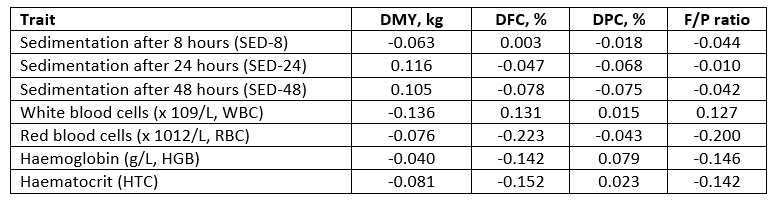
The biochemical parameters in milk were strongly (0.412-0.520), moderately (0.333-0.376), weekly (0.214-0.276) or negligibly (0.012-0.168) correlated with the daily milk traits. The F/P ratio was positively correlated with aspartate aminotransferase (AST), γ-glutamil transferase (GGT) and Ca in milk while a negative relationship existed with glucose, urea, protein, albumin and iron in milk (p>0.05).
Table 4. Correlation coefficients between milk traits and biochemical parameters in milk
*DMY - daily milk yield (kg); DFC - daily fat content (%); DPC - daily protein content (%)
The values of the biochemical parameters in the plasma due to F/P ratio classes are presented in Tab. 5. The highest values of aspartate aminotransferase (AST), γ-glutamil transferase (GGT) and Fe were determined in cows with an F/P ratio lower than 1.1 that is in acidosis risk while the lowest values were observed in cows in ketosis risk. Also, the lowest value of urea and BHB in plasma were observed in animals at risk of ketosis prevalence. Cows are ata greater risk of ruminal acidosis in early lactation, not only because of misbalance in forage/concentrate ratio but also because of the reduced capacity of absorbing short-chain fatty acids (SCFA) across the rumen due to the non-developed rumen papillae after parturition which is conditioned by the switch from high forage ratio to the high-concentrate ratio (Dieho et al., 2016). Therefore, in this research, the metabolites and parameters which could enable the prediction of acidosis risk were analysed. Higher activities of AST and GGT determined in cows marked with acidosis risk could indicate the presence of some inflammation processes in the animal's organism. More precisely, a higher proportion of concentrate could result in an increased release of bacterial endotoxin in the rumen and hindgut inducing the invasion by pathogenic microorganisms while the beneficial effects of these bacteria may be reduced (Plaizier et al., 2017; Plaizier et al., 2021). The expected milk fat depression associated with acidosis risk (Nasrollahi et al., 2017) did not occur in this research which agrees with results of Kleen et al. (2013) who reported an increased content of milk urea nitrogen (MUN) during acidosis. Stefanska et al. (2020) stated that only milk urea nitrogen could be considered as one of the indicators of acidosis occurrence in dairy cows, but the results of this research could not confirm that. Zhang and Ametaj (2017) suggested that hypoglycemia and hyperketonemia could be taken as the main marker for diagnosing ketosis.
Nowadays, other questions preoccupy researchers. For instance, how to enable early diagnose of ketosis in order to avoid the drop in milk yield or if ketone bodies are not the only metabolites altered during ketosis since multiple networks are involved in a decrease of immunity and the occurrence of inflammation? Also, cows show individual differences in the ability to process and tolerate ketone bodies (Herdt, 2000). If we use the F/P ratio as a predictor of ketosis risk, we can notice that there is a negative and weak relationship with glucose concentration in blood (Table 3) but it is not confirmed on the measured glucose concentration (Table 5). There are also some indices that hyperketonaemia increases the risk of mastitis and other inflammation (Hillreiner, 2016). The lower Fe concentration in plasma and milk in the ketosis risk group of cows determined in this research indicates some infection which corresponds to the results reported by Tsukano et al. (2019). Furthermore, besides the F/P ratio, increased content of milk protein and urea in serum could be a potential indicator for ketosis occurrence.
Table 5. LS means of the biochemical parameters in plasma in regard to F/P ratio classes
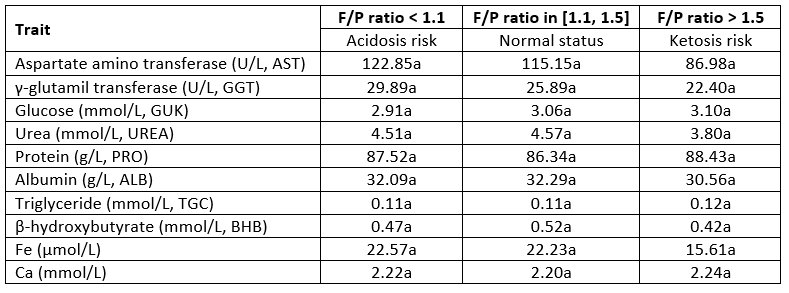
*Values within the same row marked with different letter differ statistically highly significant (p<0.05)
The sedimentation after 8 and 24 hours was highest in cows with an F/P ratio higher than 1.5, while sedimentation after 48 hours was highest in cows with an F/P ratio lower than 1.1. The highest white blood cells (WBC) were observed in cows with an F/P ratio > 1.5 (ketosis risk), while the highest red blood cells (RBC) were in cows with an F/P ratio < 1.1 (acidosis risk). The highest value of haemoglobin (HGB) was observed in animals in normal status (F/P ratio in [1.1, 1.5]). The determined differences in LS means of the biochemical parameters in plasma regarding F/P ratio classes were not statistically different (p>0.05).
Table 6. LS means of haematological parameters in regard to F/P ratio classes
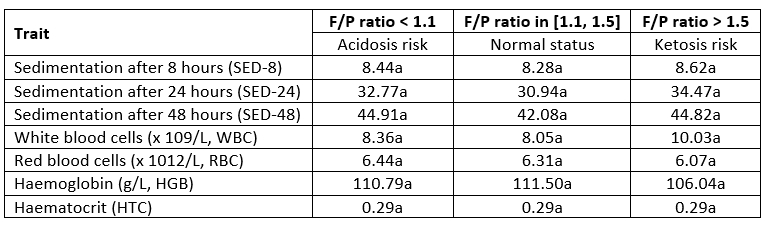
*Values within the same row marked with different letter differ statistically highly significant (p<0.05)
Opposite results to this research were reported by Marutsova et al. (2015) who found no differences in RBC in cows suffering from ketosis. At the same time, a higher WBC count in groups of cows with ketosis risk compared to normal and group with acidosis risk was determined in this research. It could be speculated that there is a risk of ketosis prevalence because some cytokines and acute-phase proteins increase in cows with ketosis (Zhang and Ametaj, 2017), predominantly interleukin-6 (IL-6), tumour necrosis factor (TNF) and serum amyloid A (SAA). On the other hand, IL-6 reduces the production of transferrin and induces hepcidin production, which blocks the action of iron transporter ferroportin 1 on the gut and, thus, reduces serum iron concentration (Nemeth et al., 2004). It has been shown that IL-6 promotes T-follicular helper-cell differentiation (Ma et al., 2012) and differentiation of CD8+ T cells into cytotoxic T cells (Okada et al., 1988) that could be comparable with higher WBC count in this research.
Table 7. LS means of biochemical parameters in milk in regard to F/P ratio classes
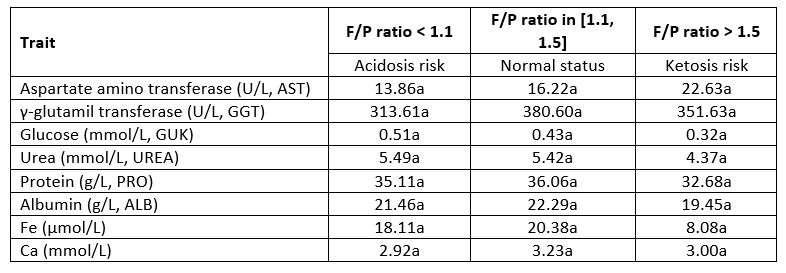
*Values within the same row marked with different letter differ statistically highly significant (p<0.05)
The highest value of aspartate aminotransferase (AST) and the lowest values of glucose, urea, protein, albumin and Fe in milk were determined in cows at risk of ketosis (Table 7). This corresponds to the results of Yameogo et al. (2008). Furthermore, the highest values of γ-glutamil transferase (GGT), protein, albumin, Fe and Ca were observed in cows in normal conditions. The highest values of glucose and urea in the milk of animals in acidosis risk indicate that those parameters, besides the F/P ratio, could indicate the acidosis prevalence. Urea in the blood is the major end product of nitrogen (N) metabolism and higher concentration suggest the inefficient utilisation of dietary N. Milk urea nitrogen (MUN) is an even better parameter because it represents the N metabolism during the entire 24 h. Usually, in cows with low rumen pH, MUN increases significantly (Stefanska et al., 2020). A similar tendency was determined in this research in a group of cows with acidosis risk.
Conclusions
According to the results obtained in this research, it could be observed that the co-variability level varies from negligible to strong depending on the set of analysed traits (daily milk traits, haematological and biochemical indicators parameters in serum and milk) of Holstein cows. The difference (but not significant) in analysed biochemical and haematological parameters due to variation in animal's health status (the F/P ratio classes indicating risk of acidosis, ketosis, or normal status of animal) were determined. Also, none of the analysed biochemical and haematological parameters could be used as a certain and accurate indicator of acidosis prevalence. Considering the obtained results, it could be assumed that besides the F/P ratio, glucose and urea in blood, sedimentation after 24 hours as well as protein and iron content in milk could be used as indicators of ketosis prevalence. It should be emphasised that the results of milk recording used as test-day records could be a valuable indicator of prevalence risk of metabolic disorders (acidosis/ketosis), while other methods should be used for unambiguous detection in situations when milk recording results indicate a risk of prevalence. Finally, this will enable a timely detection and prevention of the development of clinical forms of the disorders without additional costs.
Kovarijabilnost svojstava dnevne mliječnosti te hematoloških i biokemijskih parametara u serumu i mlijeku krava Holstein pasmine
Sažetak
S ciljem utvrđivanja varijabilnosti i kovarijabilnosti dnevnih proizvodnih svojstava te biokemijskih i hematoloških parametara, uzorkovani su krv i mlijeko 25 krava Holstein pasmine tijekom tromjesečnog razdoblja. Također, utvrđene su razlike u analiziranim parametrima uslijed zdravstvenog statusa životinje (rizik od acidoze ili ketoze ili normalno zdravstveno stanje; definirano prema omjeru mliječna masti/protein). Statistička analiza je pokazala da razina kovarijabilnosti varira ovisno o skupu analiziranih svojstava (i to od zanemarive do jake). Razlike između analiziranih biokemijskih i hematoloških parametara uslijed zdravstvenog statusa životinje nisu bile statistički značajne, ali su prisutne. Dobiveni rezultati pokazuju da se analizirani biokemijski i hematološki parametri ne mogu koristiti kao točan pokazatelj pojavnosti acidoze, dok se za indikaciju pojavnosti ketoze može koristiti sadržaj glukoze i uree u krvi, sedimentacija nakon 24 sata te sadržaj proteina i željeza u mlijeku. Nadalje, rezultati kontrole mliječnosti mogu se koristiti kao vrijedan pokazatelj rizika pojavnosti metaboličkih poremećaja (acidoza/ketoza), ali bi se druge dijagnostičke metode trebale koristiti za nedvosmisleno utvrđivanje kod životinja kod kojih rezultati kontrole mliječnosti ukazuju na rizik pojavnosti.
Ključne riječi: holstein krave; metabolički poremećaji; pokazatelji; biokemijski parametri; hematološki parametri
References
Itle, A.J., Huzzey, J.M., Weary, D.M., Von Keyserlingk, M.A. (2015): Clinical ketosis and standing behavior in transition cows. Journal of Dairy Science 98, 128-134.https://doi.org/10.3168/jds.2014-7932
