Introduction
The concept of functional foods has led to the development of varieties of food products that not only provide basic nutrients, but can also provide benefits for human health (Gouda et al., 2021; Fazilah et al., 2018; Ozcan and Karaman, 2021).
Dairy products are known for their therapeutic characteristics, including physiological and nutritional functions. These are sources of vitamins, such as A, B, D and E, and various minerals (Mostafai et al., 2019). Yoghurt is considered one of the prevalent choices and considered as a functional fermented milk product for being a good source of essential nutrients (Mudgil et al., 2018; Tomar et al., 2021). Basically, yoghurt can be classified into two groups, standard yoghurt produced from the standard starter cultures and bio-yoghurt or probiotic yoghurt, which is supplemented with probiotic cultures (Pandey et al., 2017). Probiotic yoghurt, when consumed in adequate levels (at least 106 CFU mL-1) may provide benefit effects for human health such as reduction of diarrhoea and lactose intolerance symptoms, control the serum cholesterol level due to anti-hypertensive agents (Ngongang et al., 2016) and reducing risk of colon cancers and diabetes (Barengolts et al., 2019; Rea et al., 2018). During fermentation, the probiotics play an important role in assuring the preservation of milk by producing lactic acid and antimicrobial compounds even against viruses such as SARS-CoV-19 (Gouda et al., 2021). In this environment, most of fermented dairy products are produced from cow milk. Nevertheless, it is important to use alternative milk sources for dairy products.
Camels are found in Africa and Asia, and are kept mostly by nomads and tribes living in the arid regions. The worldwide production of camel milk is in progress which was around 2700 tons of which 1092 tons were produced in Tunisia (FAO, 2019). Nowadays, camel milk production is in progress in the regions where climatic conditions make it difficult to produce bovine milk. Camel milk has specific properties like its long shelf life without heat treatment compared to cow’s milk.
The health-beneficial importance of camel milk in the human diet has been taken into account (Kaskous, 2016; Izadi et al., 2019). In addition to its higher content of essential fatty acids, camel milk contains antimicrobial agents against gram-positive and gram-negative microorganisms thus increasing its stability over time (Kumar et al., 2015). Moreover, due to the high content of antioxidant and antimicrobial compounds, camel milk supports a vital, healthy functions of the body, such as the reduction of gastrointestinal disorders, antidiabetic, anti-inflammatory, anti-hepatic, and anticancer activities (Ayyash et al., 2018; Yang et al., 2018; Soleymanzadeh et al., 2016). In general, the therapeutic properties along with antimicrobial and antioxidant effects of this dairy product are mainly attributed to the presence of different bioactive peptides derived from camel milk proteins and antimicrobial components such as immunoglobulin, lactoferrin, and lysozyme (Dharmisthaben et al., 2021). Also, many studies highlighted that the bioactive peptides derived from proteins such as αs1-, αs2-, and β-caseins play the important role in the antioxidant potential of camel milk (Izadi et al., 2019).
Several dairy products made from camel milk, including various traditionally fermented products such as koumiss, pasteurized camel milk, cheese and ice-cream have been developed and sold in many countries (Ayyash et al., 2020). Nutraceutical properties of the fermented camel milk products have been taken into consideration in some research and review articles (Ayyash et al., 2017; Ayyash et al., 2020).
Symbiotic dairy products fortified with prebiotics and probiotics continue to increase as consumers look for flavourful foods that fulfil their health needs (Balthazar et al., 2019). Prebiotics belong to the category of functional food and can be defined as the non-digestible food ingredients that beneficially affect their host by selectively providing a positive influence on the probiotic bacteria growth or activity in the colon, preventing constipation, lowering blood cholesterol and improving body defences (Balthazar et al., 2017).
Inulin is a natural prebiotic ingredient which is a mixture of fructooligo- and polysaccharides and recognized functional effects in human health. This fibre also has technological characteristics without changing sensory properties and increasingly used in many food (Heydari et al., 2017). Several studies reported that inulin was able to stabilise dairy products by the formation of soluble protein-polysaccharide complexes and the increase of viscosity (Yu et al., 2021). To the best of our knowledge, no studies are carried out to investigate in-vitro the health promoting potential of camel yoghurt (antibacterial, antidiabetic and antioxidant activities) prepared by addition of Limosilactobacillus fermentum probiotic strain.
Considering the health claims of the functional dairy market and the opportunity to advance the technology of dairy foods based on camel milk with functional characteristics, The current study attempted to highlight a new functional camel milk yoghurt and assessing the physicochemical characteristics, the functional properties including antibacterial, ⍺-amylase and ⍺-glucosidase inhibitions and antioxidant activities and the sensory evaluation of this novel product during 21 days of storage at 4 °C.
Materials and methods
Media, chemicals and probiotic growth conditions
Gelatin and date syrup were purchased from a local supermarket. MRS (de Man Rogosa and Sharpe) broth and agar (Biokar Diagnostics, France)-Vancomycin (Sigma- Aldrich, France) (20 mg L-1) were used for the selective enumeration of Limosilactobacillus fermentum CABA16 under anaerobic conditions at 37 °C for 48 h (Coeuret et al., 2003). For the enumeration of Lactobacillus delbrueckii ssp. bulgaricus, MRS Agar (Biokar Diagnostics, France) was used and the plates were incubated anaerobic conditions at 37 °C for 48 h. M17 Agar (Biokar Diagnostics, France) was used for enumeration of Streptococcus thermophilus by incubating aerobically at 44 °C for 48 h. The probiotic cells of Limosilactobacillus fermentum CABA16 were prepared by cultivating in 100 mL MRS Broth at 37 °C for 24 h under anaerobic conditions. The culture was then centrifuged (10 000 rpm, 15 min, 4 °C) and the cells were washed twice and reconstituted in Phosphate Buffered Saline (PBS) (Sigma, France). They were then served as inoculum for production of probiotic camel yoghurts.
Camel yoghurt processing
Yoghurt made of camel milk was produced according to Al-Nabulsi et al. (2015) with minor modifications. Camel milk used in this study was collected from reared camels (Camelus dromedarius) from a local farm in the south Tunisia. Briefly, milk samples were pasteurized at 90 °C for 10 min in a water bath for whey protein denaturation followed by cooling at 43 °C. The milk samples were then inoculated with 1 % of commercial yoghurt culture (Chr. Hansen, Denmark) 10 % date syrup and 0.5 % bovine gelatin (Mudgilet al., 2018).Overall, four different formulations added with or without inulin or probiotic were prepared as follows : 1) a control sample inoculated with standard cultures (CY); 2) a yoghurt inoculated with standard cultures and probiotic culture (108 CFU mL-1) (PY); 3) a yoghurt inoculated with standard cultures and inulin (5 % w/v) (InY) (Heydari et al., 2017); 4) a symbiotic yoghurt inoculated with standard cultures, probiotic and inulin (SY) followed by mixing for1 min. Camel milk samples were fermented in an oven at 43±1°C. The fermentation was interrupted when the pH reached 4.5. Finally, the samples were packaged in propylene containers (200 mL) and stored at 4 °C for 21 days. Camel milk yoghurts were sampled at 1, 7, 14, and 21 days of storage.
Physico-chemical analysis
Total acidity expressed as lactic acid percentage was determined by titrating camel yoghurt samples with 0.01 NNaOH (AOAC, 2012). The pH values of yoghurt samples were detected using a Microprocessor pH-meter BT-500 (Boeco, Hamburg, Germany). Then, the syneresis of camel yoghurts were determined as recommended by (AOAC, 2012). Briefly, 10 mL of yoghurt was centrifuged (10 000 rpm, 12 min, 4 °C) and the supernatant was recovered and weighed, thereafter, syneresis was calculated as follows:
Syneresis (%) = (W1/W2) / 100 (1)
Where: W1 = Weight of supernatant and W2 = Weight of camel yoghurt sample.
Water-soluble extract
For each sample, 10 g was mixed with 50 mL of 10 % Phosphate Buffer Saline. After that, the mixture was collected by centrifugation (10000 rpm, 5 min, 4 °C) and then kept at 45 °C in shaking water bath for 1 h. Also, the mixture was collected (10000 rpm, 15 min, 4 °C) then filtered using Whatman filter paper and the supernatant was stored at -20 °C (Van Ba et al., 2017). For each assay, the Water Soluble Extracts (WSEs) were vortexed for 1 min followed by centrifugation at 10000 rpm for 5 min.
α-amylase inhibition assay
The α-amylase inhibition assay was carried out according to the method described by Kim et al. (2004). The inhibition percentage was determined by measuring absorbance at 540 nm and calculated as follows:

α-Glucosidase inhibition assay
The α-Glucosidase inhibition assay was carried out according to the method detailed by Kim et al. (2004) with some modifications. The inhibition percentage was calculated as follows:
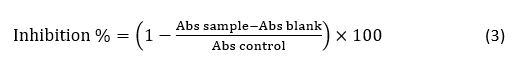
Antioxidant activity
Radical Scavenging Rate by DPPH Assay. The determination of radical scavenging activity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay was performed according to Elfahri et al. (2016). The radical-scavenging activity was expressed as follows:
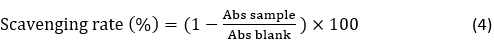
Radical Scavenging Rate by ABTS Assay. Radical scavenging rate by the 2,2’-azino-bis (3-ethylbenzo-thiazoline-6-sulphonic acid) (ABTS•+) method was determined according to the procedure of Al-Dhaheri et al. (2017). Radical scavenging activity was calculated and expressed as percentages. The radical-scavenging activity was calculated as follows:
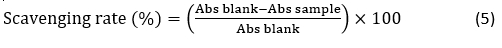
Antibacterial activity
Antibacterial ability of WSEs against pathogens such as Salmonella thyphimurium (ATCC 25922), Staphylococcus aureus (ATCC 25923), Listeria monocytogenes (ATCC 070 101 121) and Escherichia coli (DH5 alpha, Institute Pasteur of Tunisia) was determined using agar disc diffusion method previously described by Mushtaq et al. (2019). Briefly, 0.1 mL of each overnight pathogen was spotted on soft nutrient agar (Biokar Diagnostics, France) and incubated at 37 °C for 24 h. From each WSE,100 µL was put on sterile discs and plates were then incubated for 24 h at 37 °C. Diameter of clear zone around discs was measured in millimetres (disc diameter included) as its antibacterial activity.
Sensory evaluation
The assessments of colour and appearance, texture, flavour, aroma, taste and overall acceptability for all camel yoghurt samples were performed by a trained panel of 30 members using nine-point hedonic scale at 7 day of the storage (9 = extremely like, 8 = very much like, 7 = moderately like, 6 = slightly like, 5 = neither like nor dislike, 4 = slightly dislike, 3 = moderately dislike, 2 = very much dislike, and 1 = extremely dislike) (Mudgil et al., 2018).
Statistical analysis
Each sample was analysed in triplicate and the tables and the figures were averaged. SPSS statistics 20.0 commercial software was used to perform statistical analysis of the results (ANOVA) (Meyners and Hasted, 2021). Tukey test (p<0.05) significance level was performed to determine significant differences between the means. Data are presented as mean ± standard deviation. The experiments were carried out in triplicate.
Results and discussion
Acidification profile and bacterial growth
The viability of the lactic acid bacteria in different camel yoghurt formulations during refrigerated storage at day 1, 7, 14 and 21 is shown in Figure 1.
During the storage time, Streptococcus thermophilus counts were ranged, as an average, from 8.3±0.08 to 9.5±0.065 log10 CFUg-1 (Figure 1A).
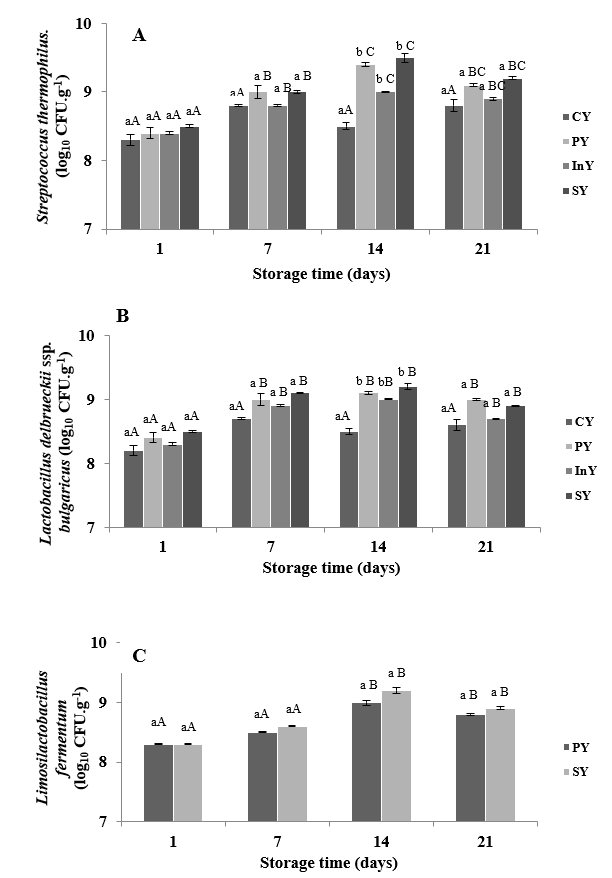
Values are mean±SD of n=3.CY: Control (Yoghurt) sample; PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added Inulin as prebiotic; SY: Yoghurt sample inoculated with added probiotic and inulin as prebiotic; Lower-case letters show the differences between the samples in the same storage time and upper-case letters indicate differences between the storage times of samples (p<0.05).
Figure 1. Microbial changes of camel yoghurts inoculated with Streptococcus thermophilus (A), Lactobacillus delbrueckii ssp. bulgaricus (B), and Limosilactobacillus fermentum (C), during storage at 4 °C
At the first day of cold storage, Streptococcus thermophilus counts varied from 8.3±0.08 to 8.5±0.02 log10CFUg-1 in CY, PY, InY and SY respectively (p<0.05). On day 14, the counts of Streptococcus thermophilus were significantly different (p<0.05). After 14 days of storage time of camel yoghurts, Streptococcus thermophilus counts decreased but it was not statistically significant (p<0.05).
Similar trends were observed during shelf-life in the counts of Lactobacillus delbrueckii ssp. bulgaricus (Figure 1B). After one week of cold storage, Lactobacillus delbrueckii ssp. bulgaricus counts varied from 8.7±0.1 to 9.1±0.14 log10CFUg-1. Numbers of Lactobacillus delbrueckii ssp. bulgaricus raised in the second week of storage to approximately 9.2±0.05 log10CFUg-1 in symbiotic camel yoghurt sample (SY) with significant difference compared to the control one (CY) (p<0.05). The addition of inulin (5%) and inoculation of probiotic bacteria did not statistically influence the Lactobacillus delbrueckii ssp. bulgaricus counts throughout the storage period in yoghurt samples, except at day 14. The bacteria counts were in agreement with observation of Alina and Kayanush (2020) and Demirci et al. (2020) who found similar trends of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus counts in camel yoghurt and yoghurt fortified with tomato powder respectively and reported that probiotics and tomato powder did not generate any statistically considerable influence on ferments yoghurt numbers.
Over, the fermented camel milks produced in all treatments (CY, PY, InY and SY) were considered to be yoghurts, the starter cultures showed satisfactory growth higher than 7 log10CFUg-1 (Codex Alimentarius, 2010).
Figure 1C demonstrates the behaviour of the probiotic bacteria in camel yoghurt treatments. Limosilactobacillus fermentum CABA16 initial value was 8.3 log10CFUg-1 for both PY and SY samples. Throughout storage, the presence of prebiotic had no significant effect (p>0.05) on the number of Limosilactobacillus fermentum CABA16. The counts of probiotic culture in camel yoghurts increased progressively up to 14 days of cold storage period and a steady decline in the numbers of Limosilactobacillus fermentum CABA16 was observed at the end of the shelf-life where the final counts were 8.8±0.014 and 8.9±0.022 log10CFUg-1 in PY and SY samples, respectively. These counts exceeded the minimum count required to confer probiotic physiological benefits (Bedani et al., 2014). Our findings are in agreement with those of Balthazar et al. (2019) who showed that inulin did not increase probiotic viability in milk-juice beverage with fermented sheep milk and strawberry. However, Costa et al. (2015) suggested that the treatment with inulin had the lowest initial value of probiotic, which an interference of this ingredient in the development of this microorganism. In fact, prebiotics may provide a positive influence on probiotic bacteria multiplication (Nurul et al., 2018).
Likewise, the present results agree with findings of Ayyash et al. (2017), who reported that the probiotic bacteria Lactococcus lactis KX881782 were maintained at >8.0 log10 CFUmL-1 in camel and bovine fermented milks. In addition, we may affirm that Limosilactobacillus fermentum CABA16 can survive in camel milk. This may be because this probiotic strain was isolated from camel milk (Mahmoudi et al., 2016).
The pH of yoghurt showed a little decrease, throughout 21 days of storage. We assessed the effect of post-acidification during refrigerated storage of camel yoghurts. Table 1 showed that pH values of camel yoghurts decreased significantly (p<0.05) during the storage period.
Table 1. Changes in pH, total acidity and syneresis of fermented camel yoghurts
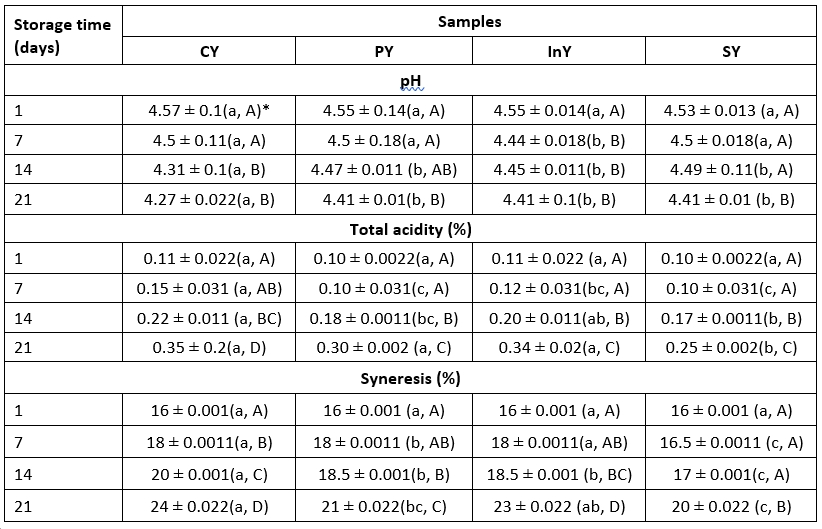
*Values are mean ± SD (n = 3); Legend: CY: Control (Yoghurt) sample; PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added prebiotic; SY: Yoghurt sample inoculated with added probiotic and prebiotic; Lower-case letters show the differences between the samples in the same storage time and upper-case letters indicate differences between the storage times of samples (p<0.05)
Initially, the pH of the freshly prepared yoghurts was found in the range of 4.55. During storage, there was slight drop in pH owing to production of acid by the action of yoghurt ferments and selected probiotic bacteria. The pH decreased from an initial value of 4.55±0.013 to 4.41±0.01 for symbiotic sample (SY) during 21 days of storage period. Consistently, TA percentage increased (p<0.05) in all samples. The observed pH and acidity values agree with Ayyash et al. (2017), who reported insignificant differences in acidity between cow and camel fermented milks respectively. Also, Kamal-Eldin et al. (2020) and Zidi et al. (2018) revealed the same pH line values of camel yoghurts.
Syneresis is one of the most visible and significant defect in yoghurts, which is due to the accumulation of whey on the surface of the yoghurt gels creating a negative impact on the consumer acceptability (Mudgil et al., 2018).
Table 1 shows the syneresis data for all samples. The syneresis values were significantly different (p<0.05) in camel yoghurts at the end of storage with maximum value of 24±0.022 % for control camel yoghurt in comparison with InY and SY. The observed behaviour was in accordance with the results of Yu et al. (2021), who studied the effect of addition of inulin biopolymer on the syneresis of probiotic yoghurt and found that addition of this compound decreased than the control sample (about 20 %). They attributed this behaviour to the interaction of inulin with the whey which facilitate the formation of gel network that can entrap the whey, leading to the increase in the water holding capacity of yoghurt. In contrast, Heydari et al. (2017) found that the highest level of syneresis was observed for yoghurts containing inulin.
Antibacterial and antioxidant activities
The inhibition ability of the WSEs against pathogens is summarized in Table 2.
The results show that the WSEs inhibited the growth of Listeria monocytogenes, Staphylococcus aureus, Salmonella Typhimurium and Escherichia coli. Antibacterial activities of WSEs were significantly higher than control (p<0.05) with a maximum inhibition diameter of 12±0.07 mm exhibited by symbiotic camel yoghurt against Staphylococcus aureus. The antibacterial activities of PY, InY and SY against all tested pathogens were not significant (p>0.05).
The antibacterial behaviour of fermented camel milk samples may be attributed to several factors such as the biofunctional properties of camel milk proteins including lysozyme, lactoferrin, and immunoglobulin, reduction of camel yoghurt pH, influence of storage temperature, and the production of antimicrobial compounds such as bacteriocins and peroxide hydrogen besides lactic acid by LAB including probiotics (Izadi et al., 2019).
Table 2. Antibacterial activity expressed as inhibition zone diameter (mm) of camel yoghurts
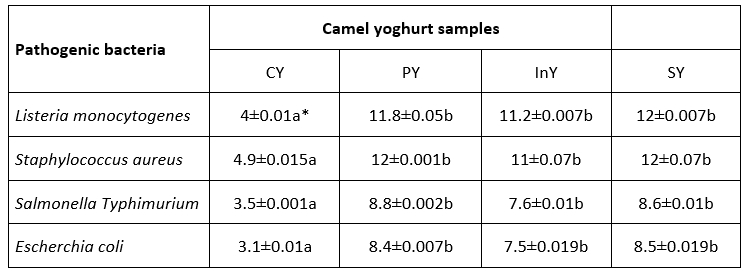
*Values are mean ± SD (n=3); Legend: CY: Control (Yoghurt) sample; PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added prebiotic; SY: Yoghurt sample inoculated with added probiotic and prebiotic; Different letters (a-c) present the differences between the samples in the same storage time (p<0.05)
Conesa et al. (2008) evaluated the antimicrobial potential of lactoferrine isolated from goat, sheep, camel, and human milks against Salmonella thyphimurium and Escherichia coli O157:H7. Results showed that lactoferrin from camel milk had the maximum antibacterial activity against Escherichia coli O157:H7. Also, Benkerroum et al. (2004) reported that Listeria monocytogenes and E. coli could be inhibited in camel milk at 4 °C by Lactoperoxydase system and lysozyme. Al-Nabulsi et al. (2015) showed that Listeria monocytogenes number was reduced in camel yoghurt during refrigerated storage. Jrad et al. (2015) also explored that the growth number of Escherichia coli could be reduced by 19.3 % in the presence of 20 g/L camel milk casein. Recently, Algboory and Muhialdin (2021) have realized that the fermented camel milk with Lactobacillus plantarum could significantly inhibit the cell growth of Escherichia coli O157:H7 and Staphylococcus aureus. Camel milk contains significantly higher concentrations of whey proteins, including lysozyme, lactoferrine and IgG which are markedly more heat resistant than their in cow milk, although the heat treatment may destroy part of the whey proteins in camel milk. Finally, the inoculated probiotic bacteria enhanced the antimicrobial activity of camel milk.
Furthermore, symbiotics have great antimicrobial properties (Cadieux et al., 2008). The combination of probiotic bacteria with prebiotic compound can cause the release of antibacterial substances such as bacteriocins, which can reduce the growth of pathogens (Fazilah et al., 2018).
The inhibitory effects of camel yoghurt formulations on α-glucosidase and α-amylase activities as indicators for antidiabetic activities through 21 days of storage at 4 °C are presented in Figure 2A and B, respectively.
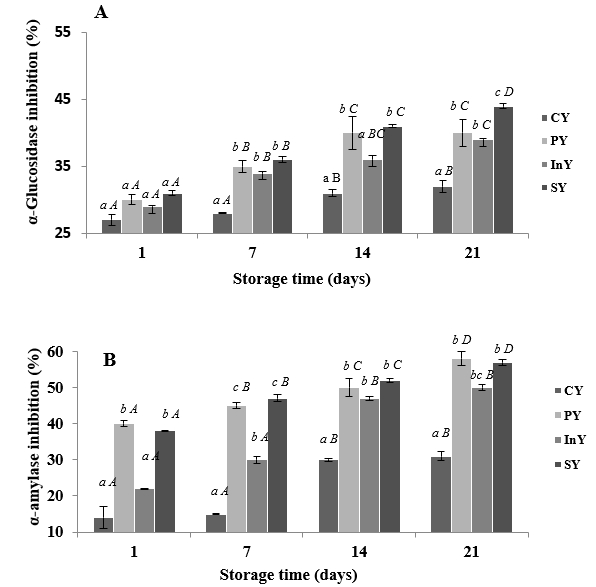
Values are mean ± SD of n = 3; Legend: CY: Control (Yoghurt) sample; PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added inulin as prebiotic; SY: Yoghurt sample inoculated with added probiotic and inulin as prebiotic; Different letters (a-c) present the differences between the samples in the same storage time (p<0.05).
Figure 2. α-glucosidase (A) and α-Amylase (B) inhibition of camel yoghurts
Noteworthy, the inhibition of α-glucosidase and α-amylase increased (p<0.05) with storage period. The percentages of α-glucosidase inhibition increased during storage and ranged from 27±0.08 to 44±0.08 % (Figure 2A). The probiotic, prebiotic and symbiotic WSEs exhibited significant α-glucosidase inhibition compared to the control during two weeks of storage (p<0.05). Similarly, α-amylase inhibitions were significantly greater (p<0.05) in PY, InY and SY samples than control during storage (Figure 2B). The PY and SY camel yoghurts showed higher α-amylase inhibition activity with final percentages of 58±2.5 and 57±0.5 % respectively.
The antidiabetic activities of different treatments were similar to those of Ayyash et al. (2017) who reported that changes in α-amylase and α-glucosidase inhibitions were important in fermented probiotic fermented camel milks. Similarly, Balthazar et al. (2019) showed that the probiotic sheep milk juice containing both fibres inulin or potato starch showed higher inhibition of digestive enzymes. The noticeable α-glucosidase inhibitions may be attributed to the bioactive peptides released by the proteolytic action of probiotics in camel yoghurts (Ayyash et al., 2018). Similarly, prebiotic addition (inulin) enhanced the proteolytic activity of this bacteria, providing more bioactive peptides in the functional camel yoghurts. Likewise, camel milk directly affects the insulin receptor function and glucose transport in the body’s insulin-sensitive tissues to regulate glucose homeostasis (Izadi et al., 2019). Overall, the high enzyme inhibitory potential of camel milk may be owing to the release of small bioactive peptides as a result of the secretion of proteolytic enzymes by probiotic strains (Kumar et al., 2016).
The DPPH and ABTS radical scavenging abilities of different camel yoghurt formulations were shown in Figure 3. DPPH radical scavenging ability increased during storage period (p<0.05) in all samples. DPPH inhibition values of probiotic (PY) and inulin (InY) enriched yoghurts and their combination (SY) were 48±2, 44±2.5 and 49±1.5 % respectively when this value was 39±1.7 % for control yoghurt (CY) after 21 days of refrigerated storage (p<0.05). In addition, ABTS radical scavenging ability of camel yoghurt samples also increased to reach a maximum of 61±1.3 % in symbiotic yoghurt. It can be seen from results that PY, InY and SY are more effective than CY for both DPPH and ABTS radical scavenging.
Antioxidant activity inhibits the oxidation of molecules caused by free radicals and is important for the shelf life of dairy foods and to protect the human body against oxidative damage upon consumption (Izadi et al., 2019). Our results are in same line as previous study demonstrated that inulin enhanced the proteolytic activity of Lp B2, producing bioactive peptides (Balthazar et al., 2019). This could be explained by crucial role of bioactive compounds in foods, in elevating the effect of reactive oxygen species such as superoxide, hydroxyl, and peroxyl radicals formed by cells under oxidative stress (Dharmisthaben et al., 2021).
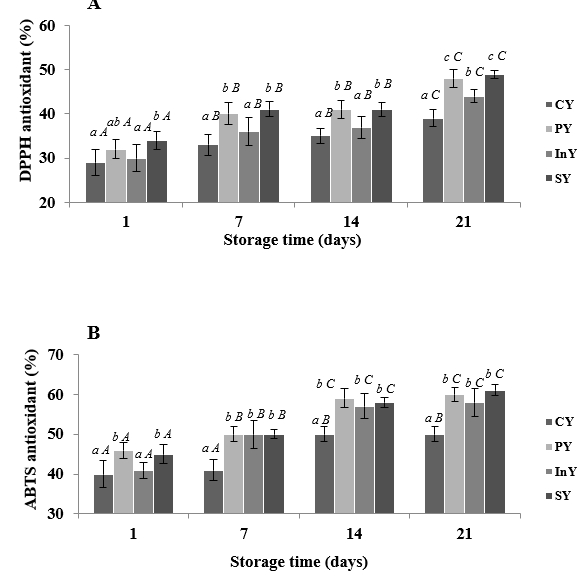
Values are mean ± SD of n = 3; Legend: CY: Control (Yoghurt) sample; PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added inulin as prebiotic; SY: Yoghurt sample inoculated with added probiotic and inulin as prebiotic; Different letters (a-c) present the differences between the samples in the same storage time (p<0.05).
Figure 3. Scavenging activities on (DPPH) (A) and (ABTS) (B) radicals of camel yoghurts
These bioactive compounds, especially protein derived peptides, can donate electrons to neutralize free radicals. Moreover, the presence of several amino acid residues in the peptide chains can enhance antioxidant properties. Moreover, the presenceof high amounts of antioxidant enzymes and non-enzymatic antioxidants(e.g., glutathione, and vitamins E and C) in camel milk can notably improve its antioxidant and antiradical activities, contributing to the control of tissue damage (Shori, 2015). Nonetheless, bioactive peptides derived from camel milk proteins (mainly αs1-, αs2-, and β-caseins) play the highest role in the antioxidant potential (Izadi et al., 2019; Oussaief et al., 2021). In consistence to our obtained results, many studies have recently interested on the fermentation role by some probiotic bacteria (include Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus kefiri, Leuconostoc and Streptococcus thermophilus, Lactobacillus acidophilus or Lactobacillus helveticus) in camel milk digestion to increase the antioxidant activity (Alhaj et al., 2017).
Inulin addition to camel yoghurts (InY and SY) significantly increased the antioxidant activity during storage compared with control (CY), showing a possible synergism between probiotic strain Limosilactobacillus fermentum CABA16 and inulin.
Sensory characteristics of camel milk yoghurt
Sensory evaluation of camel yoghurts was carried out at day 7 on a 9-point hedonic scale for colour and appearance, texture, flavour, aroma, taste and overall acceptability. Treatments have significant effects on the overall sensory parameters of camel yoghurt samples (p<0.05). Inoculation of probiotic bacteria (Limosilactobacillus fermentum CABA16) and the addition of prebiotic (inulin) had a great significance on the texture, aroma, taste, flavour, appearance and overall acceptability of camel yoghurts (p<0.05) (Figure 4). Whereas, colour was not affected by the addition of both probiotic and prebiotic. Maximum score of texture was found in the SY sample (6±0.24). Therefore, minimum scores were obtained by control sample (CY).
Values are mean ± SD of n = 3; CY: Control (Yoghurt) sample; Legend: PY: Yoghurt sample inoculated with added probiotic; InY: Yoghurt sample inoculated with added inulin as prebiotic; SY: Yoghurt sample inoculated with added probiotic and inulin as prebiotic; Different letters (a-c) present the differences between the samples in the same storage time (p<0.05).
Figure 4. Sensory profiles of camel yoghurts
The results are in same line as per previous findings of (Mohsin et al., 2019) who showed that the sensory characteristics of camel yoghurt was enhanced by biosynthesized xanthan. Moreover, Yu et al. (2021) reported that yoghurt sample fortified with inulin showed highest acceptance, flavour and texture scores compared with the control yoghurt.
Conclusions
In the present study a new functional fermented camel milk fortified with Limosilactobacillus fermentum CABA16 and inulin as prebiotic has been developed. Limosilactobacillus fermentum CABA16 number remained up to 8 log10CFUg-1during 21 days of refrigerated storage. In addition, the probiotic culture improved the physico-chemical and sensory characteristics, the antibacterial, antioxidant and antidiabetic activities of the camel yoghurt, especially when combined with inulin. Finally, the obtained results contribute to the scientific support to the process camel milk into dairy products. The presented results also encourage further investigations into the assessment of other functional properties and characterisation of the bioactive peptides derived from probiotic camel yoghurt.
Acknowledgments
This study was sponsored by the "Tunisian Ministry of Higher Education and Scientific Research" from University of Carthage. This work was performed in the research unit: Bio-preservation and valorization of Agricultural Products (UR13- AGR02) "Higher Institute of Food Industries of Tunisia- ESIAT".
Probiotički jogurt od devinog mlijeka s dodatkom inulina: antibakterijsko, antioksidacijsko i antidijabetičko djelovanje
Sažetak
Devino mlijeko poput kravljeg sadrži esencijalne hranjive tvari kao i potencijalno korisne spojeve s antihipertenzivnim i antioksidativnim svojstvima. U ovom je istraživanju korišteno devino mlijeko kao sirovina za razvoj novog probiotičkog jogurta s komercijalnim prebiotikom (inulin). U uzorcima devinog jogurta tijekom skladištenja praćene su promjene nekih fizikalno-kemijskih svojstava (pH, ukupna kiselost i sinereza) te preživljavanje bakterija uključujući i soj Limosilactobacillus fermentum CABA16. Osim toga, proizvedeni uzorci jogurta su senzorski ocijenjeni te im je određivana su antibakterijska i antioskidacijska aktivnost, kao I sposobnost inhibicije ⍺-amilaze i β-glukozidaze. Tijekom 21 dana skladištenja, pH vrijednosti uzoraka su padale, dok su vrijednosti ukupne kiselosti porasle, a soj Limosilactobacillus fermentum CABA16 pokazao je dobru sposobnost preživljavanja budući je na kraju skladištenja broj poraslih kolonija iznosio preko 107 CFU g-1. Inhibicije α-glukozidaze i α-amilaze u ekstraktima topljivim u vodi bile su veće od 35 odnosno 55 % u probiotičkom (PY), prebiotičkom (InY) i simbiotičkom (SY) devinom jogurtu na kraju razdoblja skladištenja. Štoviše, najveće antioksidacisjke aktivnosti ekstrakta topljivog u vodi (WSE) dobivenog obradom uzoraka devinih jogurta bile su oko 49 odnosno 61 % prema DPPH i ABTS testovima. Obogaćeni probiotički devini jogurti pokazali su slično antibakterijsko djelovanje na sojeve Staphylococcus aureus i Listeria monocytogenes, s maksimalnim promjerom zona inhibicije od 12±0,07 mm. U konačnici, dodatak probiotika i inulina značajno je poboljšao sva senzorska svojstva devinih jogurta, izuzev boje.
Ključne riječi: devino mlijeko; probiotičke bakterije; inulin; antibakterijski; antidijabetik; antioksidans
