Introduction
Similar to cow’s milk, camel's milk contains important nutrients as well as potentially therapeutic compounds with antihypertensive and antioxidant properties (Mahmoudi et al., 2022). Mastitis is one of the most problematic diseases in dairy animals around the world, caused by a wide range of microorganisms. In cattle, these include contagious and environmental bacteria, as well as fungi, algae, and viruses (Mbindyo et al., 2020). From the limited literature available for camels, the major bacterial pathogens isolated from subclinical mastitis were Staphylococcus, Escherichia coli, Corynebacterium, Streptococcus, Bacillus, and Micrococcus species (Rahmeh et al., 2022; Alebie et al., 2021; Seligsohn et al., 2020; Abdelgadir, 2014; Saleh and Faye, 2011).
Two main forms of mastitis could be distinguished. The clinical form is less common and is characterized by systemic symptoms in the animals and conspicuous abnormalities in the udder and/or milk. In contrast, subclinical mastitis is more common and results in decreased milk production without visible clinical signs in the udder or abnormalities in the milk. Because subclinical mastitis is difficult to assess without diagnostic tests such as the California Mastitis Test (CMT) or somatic cell counts, camel farmers pay less attention to its occurrence and consequences than to clinical mastitis (Abera et al., 2010; Volpato et al., 2015). In addition to the serious economic losses caused by the disease, mastitis has serious zoonotic potential and has been linked to the increasing development and rapid emergence of multidrug-resistant strains worldwide (Kaczorek et al., 2017; Martins et al., 2020; Algammal et al., 2020). The overuse and misuse of antibiotics is a common practice in the dairy industry and on livestock farms in general. Recent evaluations show that 56 % of farmers worldwide treat their animals with non-prescribed antibiotics (Antók et al., 2020). Careless use of antimicrobial agents and inadequate hygiene and biosecurity contribute to the emergence and spread of antibiotic resistance worldwide (Kaczorek et al., 2017).
To our knowledge, there is limited information on antimicrobial use and resistance in mastitis pathogens of dromedary camels in Algeria (Azzi et al., 2020; Saidi et al., 2021). Surveillance of pathogen susceptibility to antimicrobials is of paramount importance for animal health and public health as it provides important information for awareness, advice and policy recommendations (Acar and Roestel, 2001). Timely identification and understanding of the diversity of pathogens associated with mastitis is essential for effective control and prevention. Therefore, the aim of the present study was to determine the prevalence, identify the major bacterial causes and determine the antimicrobial profile of staphylococcal strains causing mastitis in camelids in M’sila and Biskra districts of Algeria.
Materials and methods
Study area
This study was conducted in two districts in Algeria, namely M’sila and Biskra. The study area can be roughly divided into two agroclimatic zones.
The district of M’sila is located on the semi-arid bioclimatic plain of the continental type, which is partially subject to the influence of the Sahara. The summer is dry and very hot (38 to 42 °C), while the winter is very cold (7 to 10 °C). Rainfall is very low and irregular, not exceeding 250 mm/year. Biskra is located in the lower arid or Saharan bioclimatic stage, with very irregular rainfall of less than 200 mm/year. M’sila district belongs to the central highlands region (between 34°13’ and 36°02’ north latitude; 5°20’ and 3°21’east) and covers an area of 18,175 km². It is an agricultural-pastoral district of the steppe. Biskra district is located in the southeast of Algeria (between 33°21’ and 35°17’ north latitude; 4°08’ and 6°45’ east longitude). It covers an area of 22379.95 Km². The arable area is 185473 hectares (Figure 1).
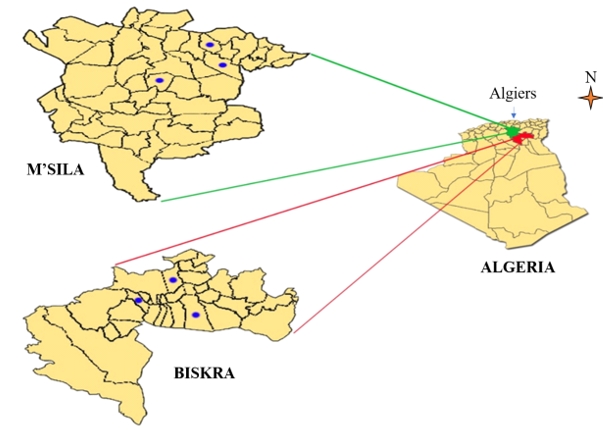
Figure 1. Geographical situation of the camel herds
Samples selection
Only herds managed under pastoralist conditions were eligible for selection. Other factors considered during selection of herds were accessibility of herds, willingness to participate and cooperation among animal owners and herders.
Ethics statement and mastitis investigation
After approval by the camel breeders, the camel udders were first visually inspected and palpated for the presence of injury, pain, heat, and swelling. Any abnormality in the color or consistency of the milk collected from each quarter was examined. In one quarter that appeared healthy after clinical examination, the California Mastitis Test (CMT) was performed in the postcolostrum period to diagnose the presence of subclinical mastitis according to the procedures of Quinn et al. (Quinn et al., 1994). A quarter that has no visible signs of clinical mastitis but shows a positive CMT result is considered to be affected by subclinical mastitis. If clinical or subclinical mastitis was detected in the udder quarter, it was sampled individually and the milk was collected according to the procedures of the National Mastitis Council (NMC, 1990). A mastitic camel was required to have at least one quarter affected.
Animal sampling
Animals were selected from thirteen dairy herds located in the departments of M’sila and Biskra in Algeria. Milk samples were collected from udder-quarters of camels with subclinical mastitis SCM, as well as from camels with clinical mastitis, just before morning milking. Teats were washed thoroughly and dried with a single-use paper towel. The first three streams of milk from each teat were discarded. The teat-end and orifice were disinfected with cotton swabs soaked 70 % ethyl alcohol and approximately 10 mL foremilk samples were collected from each quarter of camels in a sterile tube seized horizontally. Samples were immediately placed in crushed ice and submitted to the laboratory within 2-4 hours.
Microbiological analysis
Bacteriological culture was performed and evaluated according to NMC standards (NMC, 1990). Briefly, 0.01 ml of milk from each sample was plated on blood agar, McConkey agar, and mannitol salt agar. After 24- or 48-h incubation, the plates that showed one or two types of colonies on blood agar were separately subcultured onto the appropriate media for pure culture processing. A mammary quarter was considered culture positive if growth of at least one colony was detected on the strips. Samples containing more than two different bacterial species were considered contaminated.
Of the pure cultures, each isolate was identified by colony morphology, type of hemolysis, Gram stain microscopy, and various biochemical tests used to characterize the isolates. Biochemical tests included: catalase and oxidase activities, substrate utilization determined by commercial biochemical test kits, and coagulase tests using rabbit plasma to identify staphylococci. Only coagulase-positive staphylococci were subjected to Api-Staph gallery analysis. Final identification of strains was performed using the commercial API20NE kit (Biomérieux, Marcy l’Etoile, France). Several commercial API kits (API20NE, API20E, APINH, API Staph, API Strept) (Biomérieux, Marcy l’Etoile, France) were used for strain identification.
Staphylococci identification and antimicrobial susceptibility testing
In Gram-positive cocci, catalase tests with hydrogen peroxide 3 % were used to distinguish catalase-positive staphylococci from catalase-negative cocci. Coagulase tests with rabbit plasma were performed to distinguish between coagulase- positive staphylococci and coagulase-negative staphylococci. The coagulase-positive strains were further tested with the API Staph gallery assay. Antibiotic susceptibility testing for penicillin, oxacillin, cefoxitin, streptomycin, gentamicin, neomycin, erythromycin, lincomycin, tetracycline, trimethoprim, kanamycin, and norfloxacin was performed on all staphylococcal isolates using the disk diffusion methods recommended by SFM (2018).
Map conception and data analysis
The map showing the geographic distribution of herds in Figure 1 was created using Microsoft PowerPoint 2010 as available in Microsoft Office software, while the quality of the visuals was improved using Photoshop software.7.0.1 The data were presented in tables with frequencies and percentages. Statistical analysis was performed using Microsoft Excel 2016. Chi-square and Fisher's exact tests were used for data analysis and calculation of significance differences. Results were considered significant when p<0.05.
Results and discussion
Knowledge on prevalence of camel mastitis, microbial panorama, and antimicrobial susceptibility of the disease-causing agents would significantly improve prevention and guide treatment.
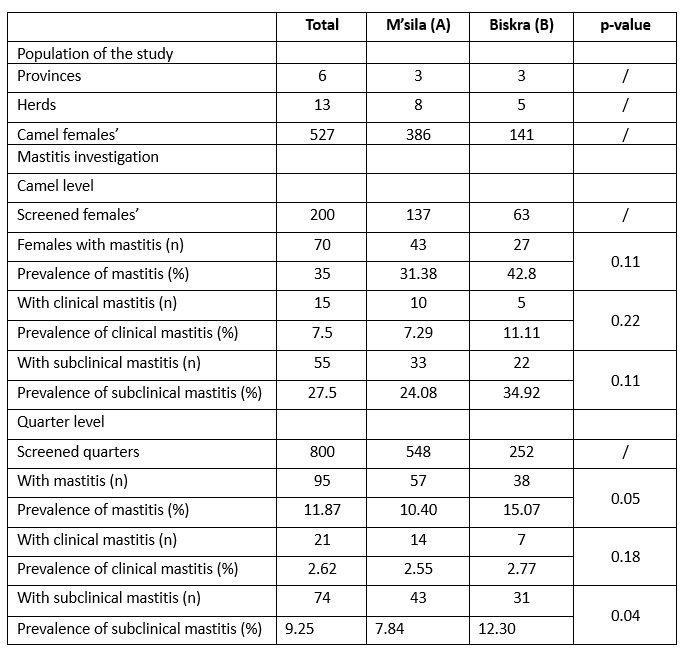
Table 1. Population of the study and mastitis prevalence in dromedary camels
Clinical and subclinical mastitis prevalence
In this survey, the overall prevalence at the camel level was 35 %, with subclinical mastitis (27.5 %) and clinical mastitis accounting for the lowest percentage (7.5 %). Of the udder quarters, 11.87 % (95/800) were affected, of which 9.25% (74/800) were subclinical. The remaining 2.62 % (21/800) were in clinical form, showing active cases of mastitis with visible signs of the inflamed udder and abnormalities in the milk (Table 1). Regarding the geographical origin, subclinical mastitis was more prevalent in the udder districts studied in the department of Biskra (p=0.04) than in the province of M’sila. The present result is in agreement with the report of Saidi et al (2021), according to which the prevalence of clinical and subclinical mastitis in Algerian camels was 3.22 % and 35.48 %, respectively, regardless of the cause. The relatively high prevalence of mastitis in camels observed in Algeria might be related to the lack of reliable mastitis prevention. Although clinical mastitis in camels is easily detected and treated, the subclinical form almost always goes unnoticed by the camel owner and spreads the infection throughout the herd (Abera et al., 2010; Alebie et al., 2021); none of the farmers routinely performed CMT or other tests to screen their camels for subclinical mastitis. In addition, the harsh sanitary conditions with limited access to water under which herders' herds operate likely play a role in the hygienic udder health complications noted in this study, as hand hygiene and milking are a cornerstone of mastitis-reducing practices (Zucali et al., 2011; Azevedo et al., 2016). Elementary milking hygiene practices, such as washing hands and udder before milking, were rarely followed in the daily routine of the herds we visited.
Increasing age and lactation stage were associated with both a higher risk of SCM and a higher risk of IMI (Seligsohn et al., 2020). As camels naturally have a lactation period of up to 24 months (Nagy and Juhasz, 2016), the spread of mastitis pathogens in camel herds could be even greater, as all herds have at least one case of mastitis. In addition, unidentified infected animals could be the reservoir for contagious bacteria; transmission can occur from one animal to another during milking through the hands of milkers (Azevedo et al., 2016; Tourette et al., 2002). The finding that subclinical mastitis is the predominant form in both camels (27.5 %) and udder quarters (9.25 %) is clear evidence of the insidious enormous economic loss suffered by the sector. This poses a serious threat to consumers, as hygienic treatment of camel milk along the dairy value chain is generally non-existent (Wanjohi et al., 2013; Jans et al., 2017). Moreover, the increase in conductivity due to intramammary infection in milk was strongly associated with prolongation of rennet addition for milk gelling (Bentayeb et al., 2023). In healthy camel milk, caseins produced by rennet-induced coagulation showed a significant change in surface composition, with a decrease in carbon and oxygen atom concentration and an increase in nitrogen atom concentration at the surface of the proteins (Bensalah and Acem, 2022). Subclinical mastitis in camels can reduce the coagulability of camel milk.
Compared to other studies on subclinical mastitis in camels, our results are higher than the prevalence of 11.67 % in camels and the prevalence of 5.86 % in udder quarters reported by Al-Juboori et al. (2013) in Abu Dhabi, United Arab Emirates, but lower than 46 % in camels and 26 % in udder quarters reported by Seligsohn et al. (2020) in Kenya. Based on our findings, subclinical mastitis was found in 25 % of camels and 8.85 % of udder quarters in Ethiopia (Alebie et al., 2021). These observed differences in the prevalence of subclinical mastitis in camels could be related to different management practises.
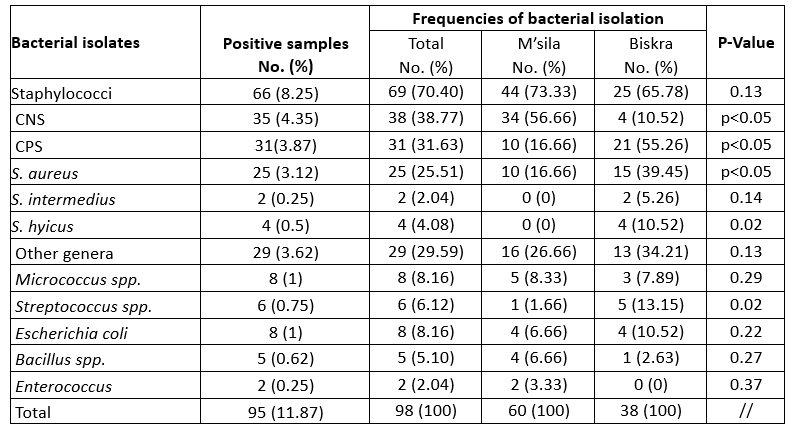
Table 2. Bacterial isolation from quarter suffering from mastitis in camel
Bacteria responsible for intramammary infection in dromedary camels
From the point of view of intramammary infection, 98 mastitis pathogens, including Gramme-positive and Gramme-negative bacterial isolates, were identified from 95 mastitic udder quarter milk samples. Staphylococci (70.4 %) were the most frequently identified isolates, with 31.63 % for CPS and 38.77 % for CNS (Table 2). Staphylococci were frequently found in milk samples from camels with subclinical mastitis as well as in cases of clinical mastitis observed in our study, which is supported by the current knowledge on mastitis-causing pathogens in Algeria (Saidi et al., 2021). Of the total number of isolates, 38.77 % coagulase-negative staphylococci (CNS) were isolated from most mastitis-positive milk samples, which is in agreement with the results of Mengistu et al. (2010) who reported 40.4 %. However, it is higher than Alebie et al. (2021) who reported 19.57 %. The high prevalence of CNS in this study could be explained by the fact that the bacterium, which is a normal skin flora, could originate from the hands of the milkers, the camel skin during milking or the camels' environment (Alamin, et al., 2013; Alqurashi et al., 2013). However, further studies are needed for Algeria to investigate the epidemiology and the specific pathogenic species involved in CNS mastitis.
In the present study, camel mastitis was mainly associated with CNS in M'sila department (p < 0.05), while a higher frequency of CPS and streptococcal strains was found in Biskra department. In contrast to CNS, which are known as facultative pathogens and are mainly isolated from subclinical mastitis cases that have no measurable impact on milk yield or clinical signs, CPS are of great importance as they are responsible for severe mastitis cases with a strong impact on milk yield and quality. The latter was found to be responsible for 31.63 % of the mastitis samples and for S. aureus in 25.51 %. The high rate of S. aureus isolation can be attributed to the fact that the skin of the udder and the milk of the infected gland are the main reservoirs of S. aureus. In addition, S. aureus can invade tissues and form deep-seated foci that are protected by a tissue barrier (Zaatout et al., 2020). The high incidence of staphylococcal mastitis may be due to inadequate hygiene, poor animal health services and lack of attention to mammary gland health in general.
Infection of udders with coliforms may be due to inadequate hygiene conditions and, to a lesser extent, faecal contamination of the udder (Geresu et al., 2021). The isolation rate of E. coli (8.16 %) in the current study relatively confirms the findings of Mengistu et al. (2010) and Alebie et al. (2021). The camels in the present study are grazed on large areas, the environment is dry and visible faecal contamination of udders, bedding and milking equipment is low.
Antibiotic susceptibility testing
Antibiotic resistance tests showed that 24 (34.78 %) of the isolates were susceptible to all antimicrobial drugs tested, while 65.21 % of the strains were resistant to at least one antibiotic. Several reports have shown that antibiotic resistance has increased among various bacterial pathogens, which has been identified as an emerging problem that raises major public health concerns due to the risk of transfer of resistance to humans and its impact on the efficacy of existing treatments (Algammal et al., 2020; Ye et al., 2017). In the present study, we described a high level of antimicrobial resistance in strains of staphylococci implicated in camel mastitis, particularly for penicillin G (33.33 %), tetracycline (31.48 %) and erythromycin (21.74 %). Resistance to erythromycin was significantly higher (p=0.03) in CPS than in CNS strains (Table 3).
Table 3. Antimicrobial susceptibility of Staphylococci involved in camel mastitis
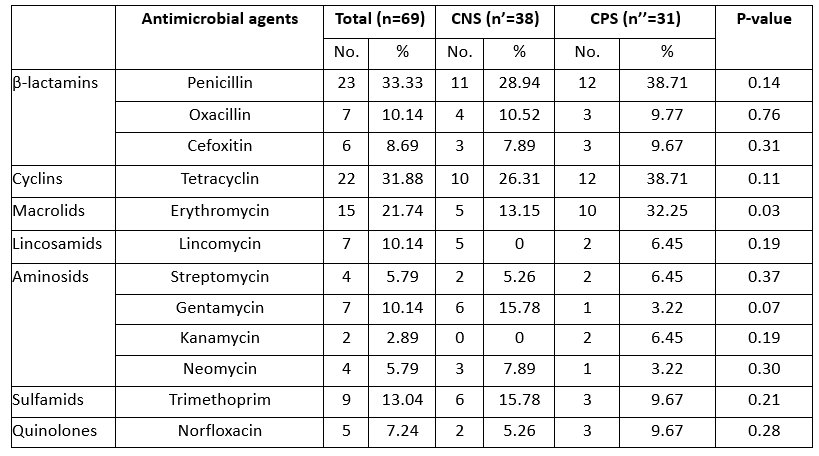
Table 4. Antibiotics resistance profiles of Staphylococci associated with camel’s mastitis
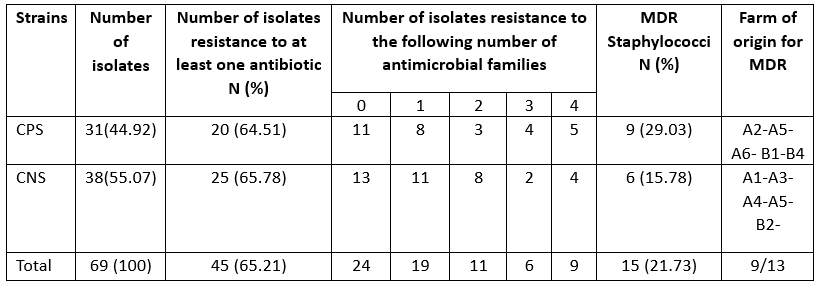
MDR: multidrug resistance if resistance to three or families of antibiotics
Similar levels of resistance to the first-line treatment with penicillin G have already been reported in strains of bovine mastitis-associated staphylococci in Algeria (Akkou et al., 2016; Saidi et al., 2015). The scientific basis for the pharmacokinetics and pharmacodynamics of antimicrobials in camels, particularly with regard to therapeutic use against mastitis, is scant (Barlow, 2011). Six isolates showed a phenotype of methicillin resistance (resistant to cefoxitin) (Table 3). Accordingly, the CA-MRSA -ST8 clone was previously reported in healthy sheep and camels in Algeria (Agabou et al., 2017). Lack of knowledge about antimicrobial withdrawal periods can lead to residues in milk, which could contribute to the spread of antibiotic resistance among dairy consumers.
Table 5. Antibiotic resistance Patterns of Staphylococci involved in camel mastitis
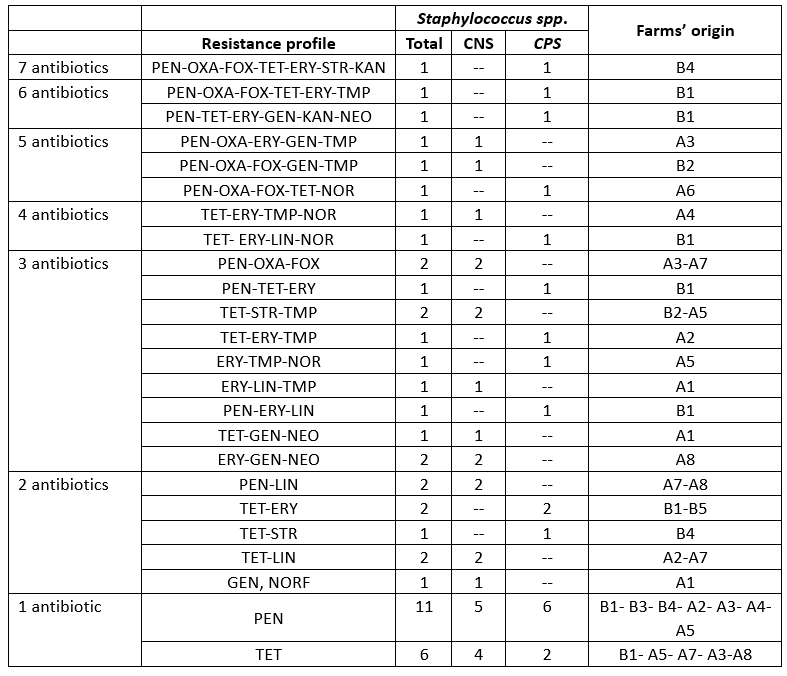
Patterns of antimicrobial resistance
Resistance to at least three classes of antibiotics is considered multidrug resistance according to the European Centre for Disease Prevention and Control (ECDC), which was found in 21.73 % of staphylococcal isolates in camel mastitis (Table 4). This indicates an alarming increase in multidrug-resistant strains of S. aureus in camels (29.03 %), which poses a significant public health risk due to the regular consumption of raw milk in the area. This result is in partial agreement with the findings of Balemi et al. (2021), who described multidrug resistance in S. aureus species. We characterised the resistant strains with 24 resistance patterns belonging to nine herds. CPS and CNS isolates showed no difference in multidrug resistance levels (Table 5).
Conclusion
The present study shows a high prevalence of mastitis, especially in subclinical forms. Staphylococci were the most common mastitis pathogen. Due to the high prevalence of mastitis and staphylococci in camels, systematic monitoring of mastitis pathogens as well as their susceptibility to antibiotics is recommended in order to introduce appropriate control measures in affected herds. The implementation of integrated approaches to mastitis prevention and control is of great importance in the context of the study in order to improve the quality of camel milk, minimise economic losses and avoid significant public health risks.
Acknowledgements
The authors extend their gratitude, especially to the pastoralists who participated in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Prevalencija mastitisa u alžirskih jednogrbih deva i antimikrobna rezistencija stafilokoka uzročnika mastitisa
Sažetak
Kako bi se istražila prevalencija, bakterijski spektar mastitisa i antimikrobna rezistencija stafilokoka uzročnika mastitisa u alžirskih jednogrbih deva, ukupno 200 ženki u laktaciji, inicijalno je pregledano na klinički mastitis, a zdrave četvrti pretražene su na subklinički mastitis pomoću kalifornijskog mastitis testa (CMT). Uzorci mlijeka iz oštećenih četvrti aseptički su prikupljeni i analizirani konvencionalnim postupcima bakteriološke izolacije i identifikacije. Zatim su izolati stafilokoka ispitani na antimikrobnu rezistenciju. Rezultati CMT-a i kliničkog pregleda deva ukazali su na ukupnu prevalenciju mastitisa od 35 % (70/200), od čega je 7,5 % (15/200) bio klinički mastitis, dok je 27,5 % (55/200) bio subklinički mastitis. Na razini tromjesečja ukupna prevalencija bila je 11,87 % (95/800), od čega je 2,62 % (21/800) bilo klinički, a 9,25 % (74/800) subklinički mastitis. Iz 95 kultiviranih uzoraka mlijeka identificirano je ukupno 98 bakterijskih izolata. Stafilokoki (70,4 %) su pri tom bili dominantni izolati, od čega je 31,63% bilo koagulaza pozitivnih stafilokoka (CPS) uključujući S. aureus s 25,51 %, a 38,77 % su bili koagulaza negativni stafilokoki (CNS). Preostali izolati bili su sojevi Micrococcus sp., Streptococcus sp., Bacillus sp., E. coli i Enterococcus. Testiranje osjetljivosti na antibiotike pokazalo je da je 24 (34,78 %) izolata bilo osjetljivo na sve testirane antimikrobne lijekove, dok su 21,73 % (15/69) bili sojevi otporni na više lijekova. Najviši stupanj rezistencije utvrđen je za penicilin (33,33 %), tetracikline (31,48 %) i eritromicin (21,74 %). Stoga je primjena integriranog pristupa u provedbi znanstvenih istraživanja od velike važnosti za prevenciju i kontrolu mastitisa kako bi se poboljšala kvaliteta devinog mlijeka, minimizirao ekonomski gubitak i spriječili značajniji rizici u pogledu javnog zdravlja.
Ključne riječi: rezistencija na antibiotike; Camelus dromedarius; mastitis; prevalencija; stafilokoki
