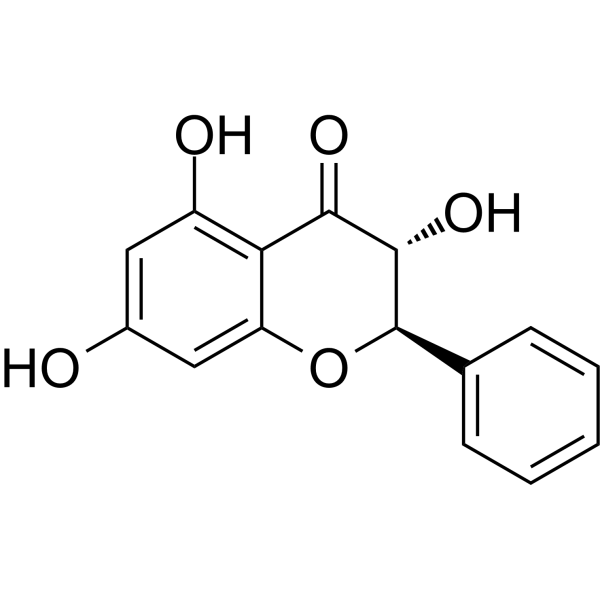
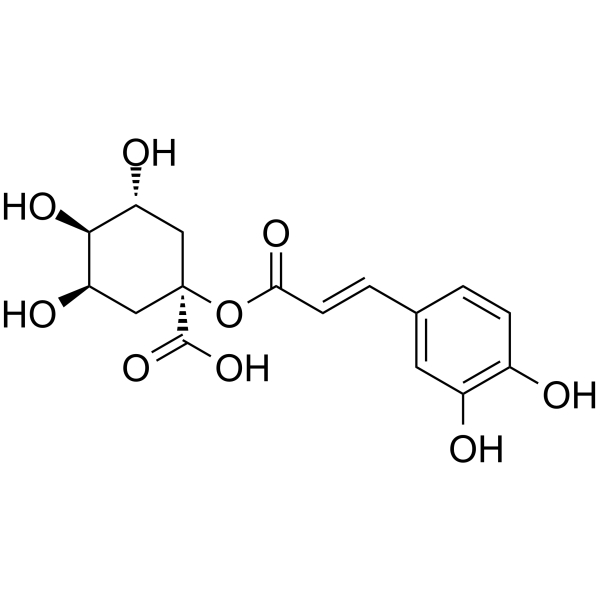
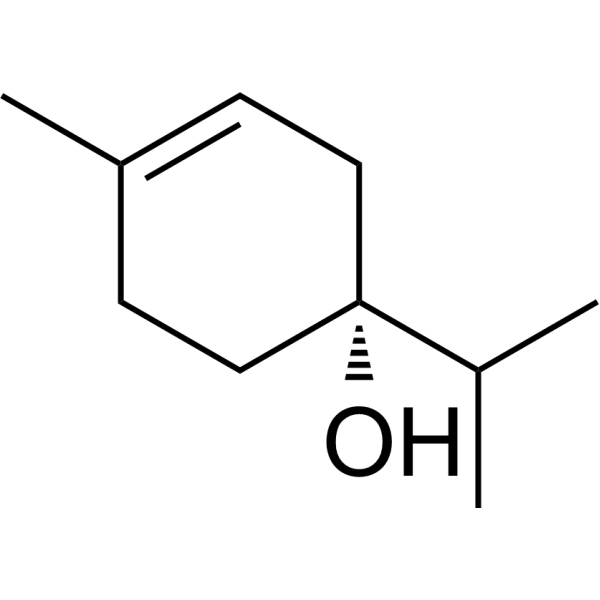
INTRODUCTION
Dry eye disease (DED) is a multifactorial ocular surface disease characterized by altered tear film homeostasis, ocular symptoms, and neurosensory abnormalities (1–3). It affects the cornea, bulbar conjunctiva, eyelid, eyelashes, and eyelid margin and its orifices (2, 3). The global prevalence ranges from 5 to 50 %, placing Europe in the middle of the spectrum (1, 3, 4). DED is a common complaint among patients and usually affects the middle-aged and older population, although its incidence is increasing in the younger population. In advanced cases, visual acuity may be reduced, and quality of life and work may be impaired (1–3). The annual cost to society is estimated at more than $55 billion in the United States (5). Individual risk factors include age over 50, female gender, hormonal changes, Meibomian gland dysfunction (MGD), Asian race, Sjögren's syndrome, diabetes, and genetic predisposing factors (3). Extrinsic risk factors include smoking, polluted or dry air, high altitude, digital device and monitor use, contact lens wear, vitamin A deficiency, graft versus host reaction, and refractive and cataract surgery (2–5). The DEWS II classification on DED is based on a reduced aqueous and lipid phase of the tear film, but in most cases, mixed forms are prevalent (2).
Ophthalmologists and pharmacists cannot ignore the expanding popularity and trend of herbal-based products entering the pharmaceutic market, since their patients suffering from ocular disorders are increasingly seeking such pharmaceutical treatments (6). As an example, in 2021, 22–85 % of Middle Eastern patients used alternative medication (6, 7), particularly for their ocular ailment, meanwhile, only 64 % of the patients were satisfied with their over-the-counter eyedrops (6). Several factors, such as the almost religious belief in "ancient" remedies, personal experiences, and preferences play a major role in the increase in demand for natural active ingredients and in the choice of therapy (8). The "Big-Pharma" conspiracy theories and the aversion to synthetic active substances can primarily be associated with more general social values, regardless of the fact that herbal extracts are largely the raw materials of our modern medicines, and scientific experiments with evidence may be behind them (7, 8).
Methodology of data collection
We conducted a literature search to find recent articles that demonstrated the therapeutic properties of medicinal plants, honey, vitamins, fatty acids, and essential oils in association with dry eye disease. The keywords (scientific botanical and English names of the known plants, “dry eye”, “blepharitis”, “herbal”, “eye drop”, “eye gel”, “eye spray”, “honey”, “propolis”, “omega fatty acids”, “vitamin”, “essential oil”, etc.) were all utilized both separately and in combinations. Commercially available relevant ophthalmic products were referred to illustrate the comprehensive review. Several internet resources were used, such as PubMed, Scopus, Google Scholar, and ScienceDirect. Any type of publication (randomized controlled trials, reviews) released between 2001 and 2024 was evaluated. All papers had been checked prior to their inclusion in this review.
DIAGNOSTICS AND PATHOMECHANISM
The tear film consists of three layers and two phases ( Fig. 1 ). The first layer, the mucin layer, is composed of glycoproteins, proteoglycans, and glycolipids, derived from the conjunctival goblet cells, the glands of Manz and the Henle crypt. It contributes to perfect refraction and visual acuity by filling in irregularities between cells (9). The second layer, the aqueous layer, makes up 90 % of the tear film and is primarily water (98 %), contains only 1 % of NaCl, KCl, ions (Mg2+, Ca2+), traces of Fe2+, proteins (0.2–0.6 %), mainly immunoglobulins (IgA, IgG, IgE), albumin, lysozyme. It is produced by the main lacrimal gland and accessory lacrimal glands – Krause and Wolfring – in the conjunctival stroma (9). The third layer, the lipid layer, and phase, is composed of apolar wax esters and polar fatty acid esters, produced mainly by the Meibomian glands. Its main function is to prevent evaporation of the tear film and protect the corneal surface from exsiccation.
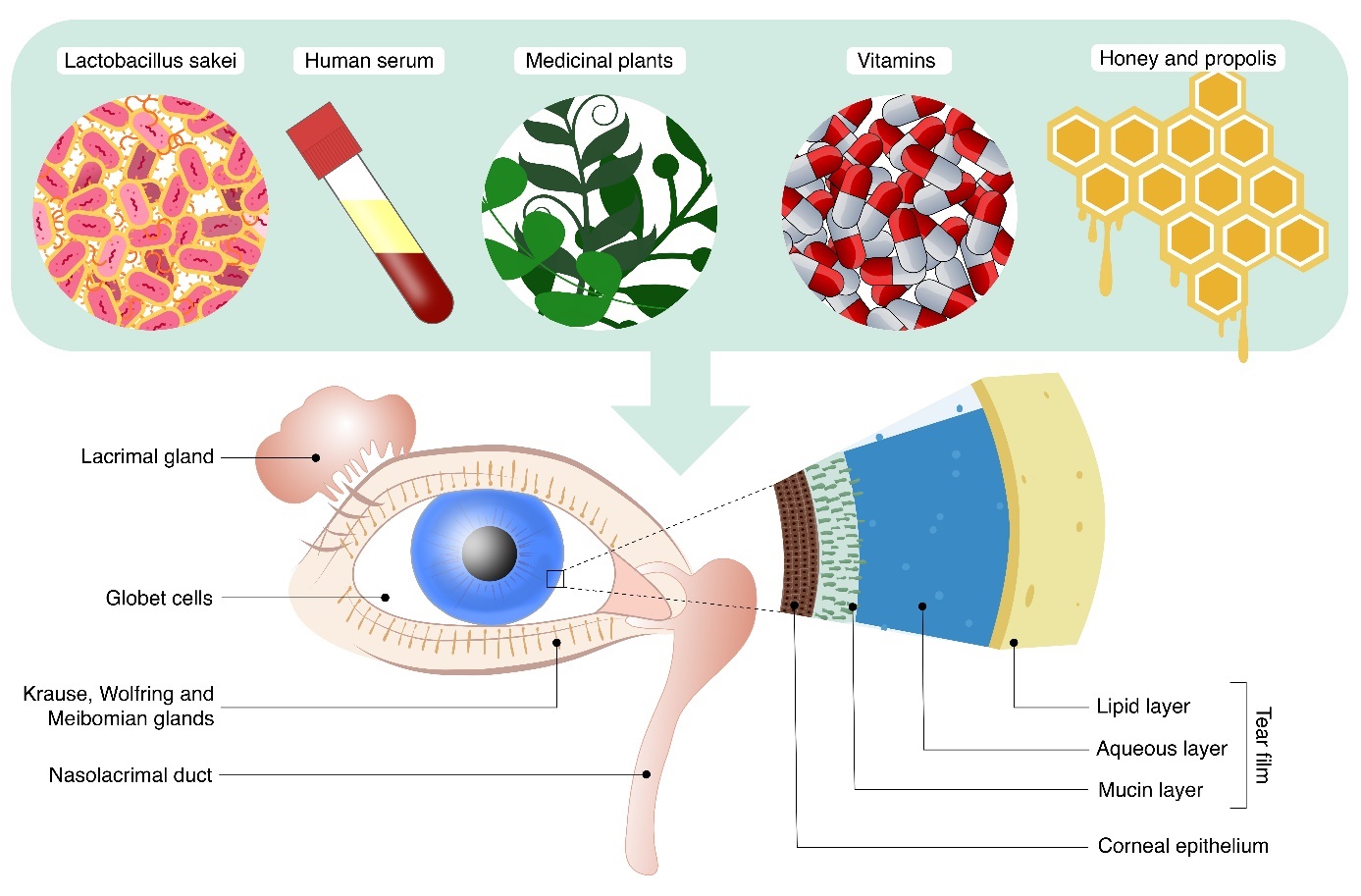
Fig. 1. Schematic figure demonstrating lacrimal apparatus and tear film constitution. The illustration provides the possible natural compounds as therapeutic materials applied in dry eye disease.
Diagnosis of DED requires understanding the patient's subjective complaints, duration, fluctuation, and possible associations with contact lens wear, visual changes, comorbidities, systemic medications, chronic use of eye drops, and living and working conditions (3). The subjective complaints are quantified by the ocular surface disease index (OSDI) questionnaire, which evaluates symptoms, environmental triggers, and quality of life impacts (1–3). Patient DED examination protocols include slit-lamp examination, diagnostic tests like Schirmer's test, tear film break-up time (BUT), staining of the cornea with fluorescein or other ophthalmic dyes (Oxford scale), tear film osmolarity, and semiquantitative tear film interferometry (1, 3). Dynamically evolving instrumentation aids in precise and accurate diagnosis of dry eye, such as the LacryDiag® device, which allows for rapid and non-invasive visualization of objective parameters of DED (1).
INITIAL STEPS OF THE STEPWISE TREATMENT
The primary approach to dry eye treatment is the topical application of eye drops, gels, and sprays to achieve tear film volume and evenness, which also increases stabilization and reduces friction between the ocular surface and the eyelids (3, 10, 11). There are several commercially available dry eye products, which differ in the main phase of the tear film they are intended to replace. Sodium hyaluronate is a glycosaminoglycan with viscoelastic properties that increases tear film stability and promotes epithelial migration. Carboxymethylcellulose (CMC) is an anionic cellulose polymer containing a carboxyl group, which has a high affinity for bioadhesion, increases tear film stability, and promotes epithelial cell migration. Liposomal dry eye treatments consist of phospholipids that enhance the lipid tear film layer and also increase tear film stability (11). Osmolarity is a key factor in ocular surface damage caused by dry eye, and artificial tears have been developed to overcome this issue (11). However, there has been no thorough clinical testing or comparative studies against other treatment options. Approximately 78 % of patients with DED have reported lipid layer deficiency, and Lee et al. (8) have suggested that liposomal spray should be the first choice for all dry eye patients. However, Moshirfar et al. (9) have set up a four-step algorithm for artificial tear drop selection that can help pharmacists and ophthalmologists in treatment selection. As a first step, CMC, hydroxypropyl methylcellulose (HPMC), and hyaluronic acid-based artificial tears were found to be the most beneficial in improving patient comfort in recent literature reviews. In the second step, polyethylene glycol (PEG) 400 and propylene glycol (PG)/glycerol-based artificial tears are considered if the patient's complaints recur refractorily. In the third and fourth steps, gel-lipid formulations (eye ointment, eye gel, liposomal spray), even hydroxypropyl cellulose-based applicators ( e.g., Lacrinsert®, Bausch&Lomb, Canada), can be added in case of chronic complaints (12). Artificial eye drop formulations are one of the longest-established first-line non-invasive treatments for the relief of DED symptoms, with annual sales of at least $540 million worldwide (2, 12). Artificial tears may alleviate the patient's subjective symptoms and improve the ocular surface condition but have no effect on the underlying pathophysiology. The volume of tear substitutes available on the market is growing exponentially (2), reaching 90 % of all ophthalmic products marketed (13), due to increasing demand and continuous development. The wide range of products available challenges both clinicians and patients when trying to choose between the available tear substitute therapies (12). Artificial tears can contain various viscosity-enhancing compounds to improve the lubrication effect on the ocular surface: CMC, HPMC, hyaluronic acid, polyvinyl alcohol, hydroxypropyl guar, and combinations of these (12, 13). Different types of "oil-in-water" emulsions containing lipids play an important role in the treatment of evaporative dry eye and MGD. There are artificial tears that contain polar phospholipids and others that deliver apolar mineral oil to the ocular surface in the form of nanoparticles. The use of preservative-free tears has paramount importance, especially in patients who are regular eye drop users due to other ophthalmic conditions ( e.g., glaucoma) (2, 10, 12). The most commonly used preservative is benzalkonium chloride (BAC), which has the highest antimicrobial activity. However, it has been shown to damage the corneal epithelium and disrupt the tear film immediately after dropping, which is directly contrary to the therapeutic purpose of artificial tears. Some of the newer preservatives, e.g., Purite® (Bio-Cide International, USA), Polyquad® (Alcon Laboratories, Switzerland), GenAqua® (Novartis Ophthalmics, USA), OcuPure® (Abbott Laboratories, USA), Dissipate® (Ocusoft, USA) have a better safety profile but do not fully prevent corneal epithelial damage (12–14). The use of preservative-containing artificial tears should be limited to 4–5 times per day, and single-dose containers are preferred due to contamination risks (12, 14). Appropriate communication is very important, as 37–53 % of patients discontinue the use of artificial tears arbitrarily due to undesired adverse effects (13) ( e.g., stinging, burning feeling, excessive tearing, or ocular surface toxicity) (13). However, a good eye drop selection is a rewarding help, as 85 % of patients are willing to pay more for a product without side effects (13).
NATURAL COMPOUNDS – A NEW TREND IN DRY EYE DISEASE
Tear substitutes used for the treatment of DED usually do not contain natural human tear components and antioxidant ingredients (15). However, they can be supplemented with herbal or other natural active ingredients and their derivatives that can enhance the therapeutic value of a particular product (summarized in Table I . Dexpanthenol, which is better known to the public, is the alcohol form of pantothenic acid (vitamin B5) (16). In addition to dermatological preparations, it is also used in ophthalmology for corneal reepithelization and hydration in the form of an eye ointment (16, 17) (Corneregel®, Bausch&Lomb, Canada). Recently, eye drops containing 2 % dexpanthenol and 0.15 % sodium hyaluronate (Bepanthol®, Bayer, Germany; and VizolS ™ Intensive, Jadran Galenski Laboratorij, Croatia) have also been introduced. Eye drops with vitamin A and vitamin E have been shown to be beneficial for conjunctival goblet cell density and corneal epithelial protection (18) (SensiVit®, Unimed Pharma, Slovakia). Vitamin A promotes corneal epithelial desquamation and sufficient ocular surface mucin production (18). Persistent vitamin A deficiency is known to cause xerophthalmia (increased ocular surface keratinization), which is now rare in the diet of developed countries but only in alcohol-dependent populations (18). Topical vitamin E (tocopherol) provides antioxidant protection and, when supplemented with coenzyme-Q, also supports corneal sub-basal nerve plexus repair (18). Vitamin E and natural triglycerides can be found in Ricinus communis L. (Euphorbiaceae) oil (19), therefore it is an ideal ingredient for ophthalmic products ( e.g., Optive Plus®, AbbVie, USA) to treat DED. Ricinoleic acid, the main bipolar molecular constituent, has the tendency to form polymers and esters, whose properties supplement and stabilize the lipid phase (19). In addition, it is a safe and effective lubricant (19). The trehalose disaccharide (3 %) active ingredient of Selaginella lepidophylla (Hook. & Grev.) Spring (Selaginellaceae) (Thealoz® Duo, Laboratoires Théa, France) was combined with 0.15 % hyaluronic acid to provide a long-lasting lubricating effect, which has also been shown in previous studies to help regulate inflammation and maintain homeostasis in dry eye patients undergoing cataract surgery (20, 21). It protects against dehydration and stabilizes human corneal epithelial cells and their cell membranes by maintaining cell hydration (22). Trehalose also provides UV-B protection to the corneal surface and promotes faster healing of ocular surface injuries (21). Ocutears® Alo+ (Santen Oy, Finland) or Visiomax™ (Eyelike GmbH, Austria), containing Aloe vera L. (Asphodelaceae) extract dissolved in hyaluronic acid (0.2 %), has shown significant clinical improvement in evaporative dry eye by reducing inflammatory mediators (MMP-9, IL-2) (17, 23). Chromone glycosides (or aloeverasides, e.g., aloesin) aid in ocular surface epithelial healing and repairment (24). In addition to its lubricating effect, Euphrasia rostkoviana Hayne (Orobanchaceae) extract (Ocuflash® Blue, Unimed Pharma, Slovakia; Artelac® Nature, Bausch&Lomb, Canada; Euphralia®, Boiron, France) has been added to artificial tears to maintain its antioxidant function and is able to stabilize hyaluronic acid by preventing UV light and ozone-induced hyaluronic acid degradation (15). Furthermore, iridoid glycosides ( e.g., aucubin) of E. rostkoviana possess anti-inflammatory and ROS-scavenging activity in human corneal cells (25). Similarly, the Plantago species [ e.g., Plantago lanceolata L. (Plantaginaceae)] also contains iridoid glycosides ( e.g., aucubin, verbascoside, and catalpol) and mucilage ( e.g., arabinose) (26, 27), which became a basis for a new eyedrop formulation (Sensivision® Plantain, Laboratoire Chauvin, France). There is an ethnomedicinal description of Plantago aqueous extract used for eyewash in case of ocular diseases, however, the wound healing properties gained more attention in traditional use (27). In a randomized, double-blind, placebo-controlled study, P. ovata Forssk. (Plantaginaceae) eye drop improved the dry eye symptoms of patients compared to placebo with the suggested anti-inflammatory and antioxidative mechanism (26). Calendula officinalis (Asteraceae) L. as a supplementary active ingredient (Ocuhyl C™, Unimed Pharma, Slovakia; Jutavit Eye Clinic™, JuvaPharma, Hungary) has been experimentally shown to inhibit bacterial biofilm formation in soft contact lenses, but no proof of efficacy in dry eye has been demonstrated (28). According to the previous antibacterial effect, Visiodoron Calendula® (Weleda, Germany) was released into the market with the indication of purulent conjunctivitis. The use of Malva sylvestris L. (Malvaceae) extract (Visiodoron Malva®, Weleda, Germany; Iridina Green®, COC Farmaceutici, Italy) as artificial tears has been shown to reduce ocular surface tension compared to hyaluronic acid, which may result in proper eye drop distribution and prolonged eye hydration (29). The naturally occurring mucilages ( e.g., trehalose) and flavonoids ( e.g., malvidin) contribute the prolonged hydration (30). In addition to its chemical properties, M. sylvestris has pharmacological actions that modulate cytokine production, promote anti-inflammatory activity, regenerate ocular surface epithelium damage, and promote lipid accumulation in Meibomian glands, making it an ideal artificial tear substrate (31).
APITHERAPY – APPLICATION OF BEE PRODUCTS
Apitherapeutic products, such as honey, propolis, or royal jelly, have long been used as an ancient remedy for natural wound healing due to their antibacterial, antioxidant, and anti-inflammatory properties (7, 32), and the concept of using the medicinal honey of the Leptospermum spp. trees (Myrtaceae) from New Zealand (manuka honey) as an artificial tear ingredient have been previously proposed (33, 34). The active ingredients mainly pinobanksin, pinocembrin, and chrysin are responsible for the previously mentioned positive medicinal effects (32). For evaporative dry eye, contact lens dry eye, and MGD, studies have been conducted on a 16 % manuka honey-containing artificial tear approved in Europe (Optimel Manuka+ Dry Eye Drops™, Melcare Biomedical, Australia). Manuka honey is discovered in Australia and New Zealand and is an increasingly popular specialized medication, with the only negative drawback of its significantly high cost (33, 34). TFOS DEWS II mentions a dry eye model, in which oral royal jelly could restore tear secretion, but the exact product or management strategy has so far not been elaborated (35). Manuka Honey CycloPower™ microemulsion eye cream for blepharitis on human and rodent preclinical subjects confirmed a safe antimicrobial activity in inhibiting bacterial growth and improved lipid layer thickness. Bee-based compounds, such as propolis (7) can be combined with dexpanthenol and other herbal extracts [Matricaria recutita L. (Asteraceae) and Aloe vera L. (Asphodelaceae)] to gain a novel product e.g., Oculocin® Propo (Origmed, Lithuania) eyedrop. Even though propolis possesses antimicrobial and anti-inflammatory properties (7), due to its flavonoid and phenolic acid content (such as 1-caffeoylquinic acid) (36), no randomized controlled research has been conducted on its effectiveness against DED ( Table I.
Finally, it is worth mentioning, that applying “home-made” unprocessed honey products on the ocular surface, instead of sterile pharmacologically processed honey-based eyedrops, is strictly prohibited. Corneal ulcers caused by Acanthamoeba keratitis and Trichophyton mentagrophytes fungal keratitis were already reported after a traditional healer’s self-remedy suggestion using unprocessed honey on the ocular surface (37).
HUMAN TISSUES AS NATURAL SOURCES
Eyedrops of natural origin include autologous serum tears, which have the advantage of possessing many biochemical properties similar to human tears [pH, vitamins, fibronectin, epithelial and neuronal growth factor (EGF-NGF) content, etc.]. The autologous serum, prepared from the patient's own venous blood by phoretic or ultracentrifugal techniques with appropriate sterility and dilution of 20–50 %, promotes the regeneration of corneal epithelial cells, increases the number of goblet cells, and inhibits the release of several pro-inflammatory cytokines. Patients' tear film stability significantly improved, with achieved higher OSDI scores by the application (2, 22). Its widespread use is currently hampered by its production cost and difficulties in keeping it sterile and stable ( Table I.
New innovations targeting other promising human tissues, such as the amniotic sac, contain amnion and chorion derived from the placenta's internal layer (38). The amniotic membrane comprises a large extracellular matrix, which includes collagen, laminins, cytokines, and growth factors. It possesses anti-inflammatory, anti-angiogenic, immunomodulatory, antibacterial, anti-scarring, and hemocompatibility characteristics. Similarly to the autologous serum artificial tears, this solution is also rich in fibronectin, growth factors, and collagens as effective sources of ocular surface regeneration. Several companies manufacture ophthalmic solutions from amniotic membrane extract, which can be coupled with umbilical cord blood to create another type of remedy, such as Regenesol™ (Biotissue/Tissue Tech Inc., USA), Genesis Amniotic Cytokine Extract (ACE)™ (Ocular Science Inc., USA), and Regener-Eyes™ (Regenerative Processing Plant, USA). They have the potential for expanded clinical application in ocular surface disorders like DED, but more clinical trials are required to determine consistency and efficacy ( Table I.
BEHIND THE SYMPTOMS: THE MICROBIOME AND INFLAMMATORY THEORIES
Anti-inflammatory and immunosuppressive agents can be effectively used to reduce ocular surface inflammation and increase tear production, which is the cause and effect of DED: an eye drop containing cyclosporin A at a concentration of 1 mg mL–1 in the form of a cationic nanoemulsion (Ikervis®, Santen Oy, Finland) has been available in Finland since 2017 for the off-label treatment of severe corneal inflammation associated with dry eye (2, 10, 22). The preservative-free topical steroid hydrocortisone (Softacort®, Laboratoires Théa, France) with a concentration of 3.35 mg mL–1 is also an excellent treatment for ocular surface inflammation with a short 2-week application (2, 10, 22, 39). They reduce the inflammatory biochemical cascades and the gene expression pathways that induce and control them. Given their low intraocular penetration, negligible intraocular pressure elevation, and cataractogenic effects, they are safer to use with a significantly better side-effect profile than the currently available topical steroids. Cyclosporin A 0.05 %, tacrolimus, and lifitegrast 5 % inhibit apoptosis of ocular surface cells induced by inflammatory biochemical pathways and migration of T lymphocytes to the ocular surface, but they require a longer time course compared to corticosteroids before they become effective in controlling inflammation (4, 10, 22). Systemic side effects are not expected due to the low absorption potential across the ocular surface mucosa, and a local unpleasant burning sensation may occur in some patients (10).
Dry eye may also be associated with anterior blepharitis, demodicosis blepharitis caused by Demodex mite infection (3, 17). There are also several methods for managing MGD, which causes and/or is associated with significant dry eye symptoms. Gentle lukewarm towel massage of the Meibomian glands and eyelid hygiene procedures have been shown to be effective in the management of clinical symptoms of chronic blepharitis, including associated dry eye complaints in adults and children (17, 22). A wide range of product families containing herbal active ingredients are available to patients, e.g., Melaleuca alternifolia L. (Myrtaceae) oil (Puralid® Lipogel, Santen Oy, Finland) and Calendula officinalis L. (Asteraceae) extract (Blepharolotion™, Geltec-Medica, Russia). Innovative solutions are offered by products containing M. alternifolia oil (Ocuvane® Plus, SolMed Pharma, Hungary), C. officinalis extract (Blepharowipe™, Geltec-Medica, Russia) (17) and ozonized vegetable oil (Ozolid®, FB Vision, Italy), which can be unfolded from sterile packaging and wrapped around the fingers for easy care of eyelids and eyelid tissues, similar to eyewashes. Terpinen-4-ol (2–4 %) from M. alternifolia was claimed to be effective against eyelid demodicosis (40), while the numerous bioactive components of C. officinalis (with α-cadinol, as the major constituent of the plant), has an excellent anti-microbial effect (17, 26, 41, 42). In addition to blepharitis, various eyelid pathologies can also lead to DED (such as atopic dermatitis, periorbital eczema, etc.) (17). The studies from Alantel aimed to develop a natural, nourishing, and moisturizing cosmetic cream originally for the prophylaxis and treatment of mild dermatitis induced by radiation therapy in breast cancer patients (43, 44). The novel herbal combination (Alantel®, Nakafarma, Spain) of Aloe vera L. (Asphodelaceae), Matricaria recutita L. (Asteraceae), and Thymus vulgaris L. (Lamiaceae), contains antibacterial ingredients to reduce redness and irritation (chamazulene, thymol, carvacrol), promote the local dermal immune system (carricine), combat immunosenescence, and promote the healing of epidermal lesions (phytosterols, mannose phosphate), however, atopic consequences of antiglaucomatous eye drops and other irritative eyelid pathologies are also the therapeutic target of the product ( Table I).
Furthermore, tetracycline and its derivatives ( e.g., doxycycline, minocycline), administered as oral tablets, reduce the amount of lipolytic exoenzymes produced by bacteria, and thus the toxic and irritative free fatty acidic products released during the pathological degradation of meibum (the lipid-containing secretion secreted by the Meibomian glands) (2, 10, 17, 22). By reducing the activity of collagenase, phospholipase A2, and matrix metalloproteinases, they inhibit inflammation of the corneal epithelium and ocular surface, thus improving the symptoms of MGD and anterior blepharitis, which are primarily associated with rosacea and secondarily cause dry eye complaints.
The ocular surface microbiome is also crucial in DED pathophysiology, as it shows different responses to bacteria. Several studies demonstrated gut microbiome alterations in auto-immune diseases associated with dry eye symptoms, making it a potential therapeutic target of pre- and probiotics. The conjunctiva-associated lymphatic tissue (CALT) tolerates some microorganisms, known as leading to improved symptoms and suppressed inflammatory responses that leave on the ocular surface as resident microbiota or normal flora. Postbiotic ophthalmic formulations containing Latilactobacillus sakei lysate (Immunodrop sakei™, FB vision, Italy) significantly improved the signs and symptoms of DED and suppressed ocular surface inflammatory response (4) ( Table I).
THE CRITICISM AND LOW EVIDENCE BEHIND SOME HERBAL FORMULAS
The above-mentioned examples present that herbal products are becoming more and more prominent due to patients' increasing preference for natural active ingredients (7), which is not negligible in the choice of artificial tears for DED patients. However, it is worth mentioning that some products contain herbal active ingredients that have not been tested in DED or other ophthalmic patient populations (summarized in Table II ). Their pharmacobotanical effects have not been exactly proven in ocular diseases yet or are included neither in the European Medicine's Agency (EMA) phytotherapy guidelines, nor the European Pharmacopoeia. This just partly applies to Hamamelis virginiana L. (Hamamelidaceae) (17, 45–48), which is the raw material for several artificial tears ( e.g., Iridina Green®, Jutavit Eye Clinic™) or eyelid products (Blepharolotion™ and Blepharowipe™) on the market in the European region. Makarov et al. (17) mention the high flavonoid and tannin content of H. virginiana plant, which theoretically might contribute to antibacterial and vasculoprotective effects on eyelids, but there is neither ethnomedicinal nor scientific evidence of its ophthalmic efficacy. Although H. virginiana distillate is given in the EMA monograph (application as a traditional herbal medicinal product in the form of eye drops) (45), EC (traditional herbal medicinal product to be used for the temporary relief of eye discomfort due to dryness of the eye or to exposure to wind or sun) (46), and Ph. Eur. or individual drugs and dosage forms (47), and mentioned in USP 2024 for distillate from dried twigs and extraction solvent (48), the authors did not find any relevant scientific validation. One should also be critical when there is ophthalmic evidence for an herbal extract, but the available product does not represent the correct pharmaceutical form. For example, the beneficial effects of Vaccinium myrtillus L. (Ericaceae) extract have been studied in retinal conditions ( e.g., glaucoma, age-related macular degeneration) (7, 49). More recently, measurable improvement with OSDI has been found in DED, but the applicability has so far been limited to oral supplements only (49). Although there is a product containing such an extract on the market (Ocutein® Sensitive Plus, Simply You Pharmaceuticals, Switzerland), no topical application has been studied, so there is no evidence that what is effective orally can be equally effective through ocular surface structures. Echinacea purpurea (L.) Moench (Asteraceae) meristematic stem cell extracts are also ingredients of some commercially available eyedrops (Ekidrop™, Soleko, Italy), which have recognized moisturizing properties, emollient phytosterols, nourishing amino acids and mineral salts necessary for osmotic balance (50). The plant is popularly used for enhancing the immune system functions and prevention of the common cold (50, 51), on the contrary, its oral use has been associated with DED development (51); topical application has not been evaluated. Fermented extract of Mercurialis perennis L. (Euphorbiaceae) has been used in European folk medicine for its wound-healing properties and in irritative conjunctivitis (52). However, there is only one product in the market (WALA® Mercurialis Augentropfen, Wala International, Germany) containing M. perennis and essential oil of Rosa × Damascena Mill. (Rosaceae). According to the package leaflet, the product stimulates tear production in the eye in case of sicca conjunctivitis (possible aqueous secretagogue), however, no clinical trial was conducted to prove its efficacy.
According to Bürgi's law, multiple herbs provide greater pharmacological action through synergism than if administered independently (7); therefore clinical testing of polyherbal eye drops is challenging. Furthermore, it is debatable whether such a large number of herbal elements is actually necessary, as the same effect might be accomplished with fewer active compounds. As multiherbal products, Iridina Green®, Gabriel® and Navi®Infla) eye drops are commercially available in the European pharmaceutical market. Navi®Infla is formulated with more exotic ingredients: Rosa × Damascena Mill. (Rosaceae), Phyllanthus emblica L. (Phyllanthaceae), and Curcuma longa L. (Zingiberaceae) which have a strong antioxidant activity useful in relieving oxidative stress discomfort thus helping to reduce the symptoms of ocular surface inflammation. The aforementioned plants are an integrative part of traditional Ayurvedic eye drop formulations. Ophthacare® (Himalaya Wellness, India) containing basic Ayurvedic herbs including Trachyspermum ammi (L.) Sprague ex Turrill (Apiaceae), Terminalia bellirica (Gaertn.) Roxb. (Combretaceae), Phyllanthus emblica L. (Phyllanthaceae), Curcuma longa L. (Zingiberaceae), Ocimum tenuiflorum L. (Lamiaceae), Camphora officinarum Nees (Lauraceae), Rosa × Damascena Mill. (Rosaceae) and processed honey (53). Ophthacare® was useful in an open prospective multicenter clinical trial which was conducted in patients suffering from various ocular surface disorders and postoperative prophylaxis after cataract surgery. Similar polyherbal eye drop (Itone™, Dey’s Medical Stores, India), a mixture of aqueous distillates of nineteen traditionally used Ayurvedic ingredients; anti-inflammatory, antioxidant, and even anti-cataractogenic properties are claimed (54), even though no controlled experimental studies have been performed.
Furthermore, it is also worth investigating whether eye drops containing a mono- or polyherbal component are beneficial in the treatment of DED, whether there is an interaction between them, or if there is a significant difference in their preparation. Knowing the wide range of contraindications of Hypericum perforatum L. (Hypericaceae), the question is obvious, how effectively it does weaken the biological effect when formulated with other herbal components? In addition, artificial tears containing Hypericum perforatum ( e.g., Lacrimal® Natura, Polpharma, Poland) may also be a contraindication for glaucomatous DED patients on topical beta-blocker therapy, such as dietary supplements containing its extracts (7).
NEW STRATEGIES COMBATING DRY EYE
Liposomal eyelid sprays are the preferred first-choice administration route for patients with fear of eye drop instillation or difficulty in self-management (11). Hippophaë rhamnoides L. (Elaeagnaceae) and hyaluronic acid-containing eyelid spray (BioDrop MD® Spray, Piiloset, Finland) showed significant improvement in dry eye symptoms compared to a commercial reference spray and untreated control eyes over a 45-day treatment period without adverse effects (55). H. rhamnoides contains essential fatty acids (α-linolenic acid and linoleic acid), which can be converted into precursors for eicosanoids, which regulate inflammation. The nasal route of administration is another recent pharmacotechnologic development. The U.S. Food and Drug Administration (FDA) has approved the first varenicline solution nasal spray (Tyrvaya™, Oyster Point Pharma, USA) to treat DED. The nasal spray introduces a novel delivery method as well as an alternate therapy technique. It stimulates the nerve connected to tear release and stimulates the lacrimal and Meibomian glands (56). This may include people who opt for a non-ophthalmic method, those who have trouble delivering topical eye drops, and contact lens users who must coordinate ophthalmic administration with contact lens usage. The new treatment approach also favors the development of herbal medicines, such as in a randomized, double-blind, placebo-controlled study on intranasally administered Viola odorata L. (Violaceae) oily extract enhancing neural tear production and improvement of tear film stability (57). In the latter case, product development has not been implemented, but it is predicted that medicinal herbs will eventually be employed for all novel pharmacological innovations.
LOW EVIDENCE COMPLEMENTARY STRATEGIES
The study of Kuo et al. (58) on DED (the exact method of randomization and blinding was not disclosed) found that the application of Salvia sclarea L. (Lamiaceae) and Aniba rosaeodora Ducke (Lauraceae) essential oils massaged around the eyes for 5 to 6 minutes, twice weekly for 8 weeks, improved symptoms such as lipid layer quality, blinking quality, tear meniscus height, and noninvasive tear break-up time comparing to the placebo [ Vitis vinifera L. (Vitaceae) vehicle oil] or no treatment group. The exact mechanism, pharmacological pathway, and optimal dosage are not elucidated yet. The authors suggest the role of linalool by regulating the periocular autonomic nervous process to enhance tear secretion.
Acupuncture, a Traditional Chinese Medicine treatment for a variety of conditions, has been shown to enhance symptoms and tear volume in dry eye patients. Acupuncture, including laser acupuncture and silver spike point electrotherapy, has been demonstrated in studies to improve tear secretion and reduce symptoms (14). However, the effectiveness of acupuncture in treating DED is often based on artificial tears, rather than sham needling (14). In one randomized clinical trial, subjects with DED reported significant improvement in symptoms after four weeks of acupuncture, indicating that both sham and true acupuncture are equally effective in treating DED symptoms (14). Acupuncture at the BL1 point (Jīng míng) can significantly improve lacrimal secretion and symptoms of moderate and severe dry eye disease (DED), with a better curative effect than artificial tears (59). BL1 point neuroanatomically belongs to the trigeminal nerve distribution in the ophthalmic nerve division. Treatment-related adverse effects (8.3 %) included mild bleeding, which was resolved quickly by a cold compress. The study had limitations, including not limiting DED subtypes, not performing blinded methods, and not considering psychological expectations. Future trials should stratify participants, consider placebo control, and investigate participant expectations to avoid bias and improve results.
Low evidence-based treatments, including many human and veterinary disorders, cannot be ignored in the case of DED, as well. Pilot studies or animal model experiments have been conducted in certain cases, but multicentric randomized clinical trials are frequently inadequate, and many of them are based on unvalidated personal experience.
TEAMWORK IS ALWAYS ESSENTIAL
In cases where conservative therapies fail, invasive methods performed by ophthalmologists like correction of eyelid malposition, surgical closure of eyelids, tear plugs, thermocoagulation, amniotic membrane transplantation, or salivary gland autotransplantation can be used as a therapeutic step (2, 14). In conclusion, the scientific literature also concludes that in patients with DED, regular use of artificial tears, regardless of the preservative content, will definitely result in a significant improvement in quality of life (12). In addition, it is essential to promote patient education and to modify environmental or lifestyle factors that can be implemented to reduce the symptoms of DED. Nutritionists can also have a role in dietary modifications and suggestions for DED patients. In addition to plenty of fluid intake, the diet can be supplemented with daily 2 × 15 mL of Olea europea L. (Oleaceae) oil or omega-3 fatty acids, which inhibit the release of inflammatory mediators, suggested by the DREAM (Dry eye assessment and management) study (10, 22). Makarov et al. (17) suggested avoiding eating spicy, salty, and fried meals, however, the evidence is not clear. It is worth pointing out the importance of taking regular breaks (“20-20-20-rule”) from excessive use of digital devices (computer vision syndrome) to rest the eyes ( e.g., blinking, looking into the distance, "palming", "ocular yoga", “scanning”, etc.) every 20 minutes, at least 20 feet away, for at least 20 seconds (60, 61). The office exercises were elaborated and patented by Kolpakov et al. (62) (also known as Kolpakov’s gymnastics) over 35 years ago, and they were tested on workers from various firms in Moscow to treat the asthenopeic symptoms of near work by stimulating systemic and ocular circulation. Applying the latter-mentioned methods in everyday life can be useful not only for ophthalmologists but also for visual trainers and optometrists.
Empathy and taking the time to explain their illness to our patients, who can often become isolated without much understanding from health professionals and/or their relatives, is an important part of the treatment process. After a while, patients may feel that their illness is unmanageable, leading to a decrease in compliance and discontinuation of treatment. This can ultimately perpetuate the vicious circle of ocular surface damage (13, 22). For physicians and pharmacists, it is important to explain to the patients that their disease is chronic and that the severity of symptoms may fluctuate depending on the body's internal conditions and reactions to environmental conditions (22). The role of psychologists and psychiatrists or emergency helplines should not be underestimated. Anxiety and depression are 1.82 times more prevalent in patients with DED than in healthy controls (63). It has a significant effect on quality of life, moreover, DED is also linked to an increased incidence of suicidal thoughts. Prevention, education, and advice are also important tasks for other professionals such as optometrists, opticians, and rehabilitation eye trainers, facilitating the work of ophthalmologists and pharmacists (61). Therapies should be regularly monitored by ophthalmologists and modified if necessary. Although longer time and patience are required, personalized DED management can significantly improve quality of life (22).
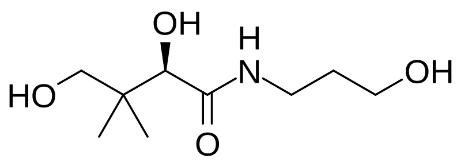
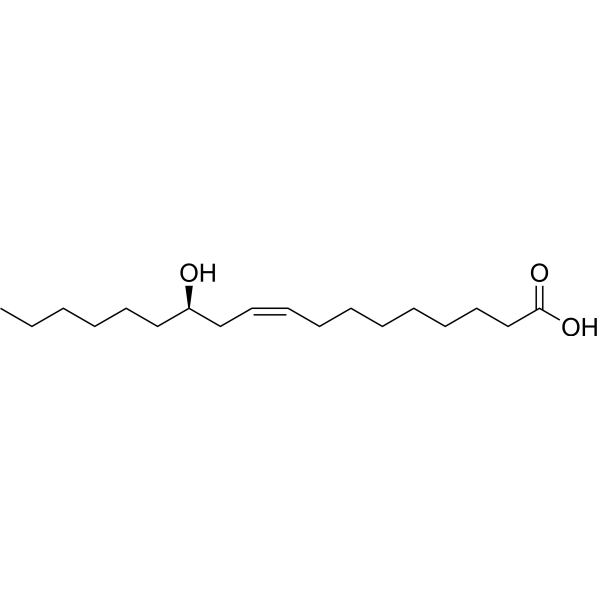
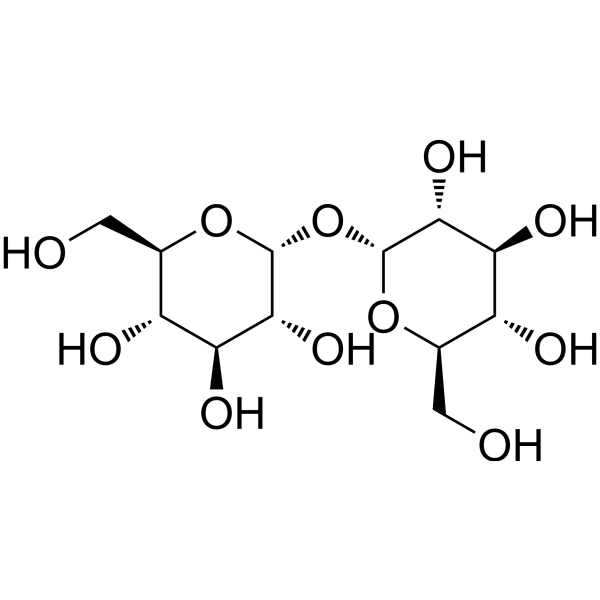
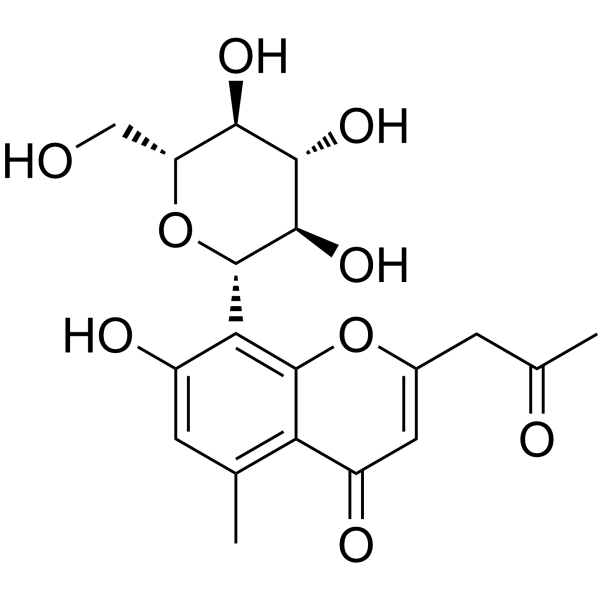
Table I. Self-edited summarizing table about the existing products treating DEDa
DED – dry eye disease
a Information sources are detailed in the main text and references. Source of the molecular structures are derived from MedChemExpress.com.
b Chemical structure presented.
Table II. Self-edited summarizing table about the contradictory products and methods associated with DEDa
a Sources are detailed in text and references.
CONCLUSIONS
Dry eye disease is a multifactorial ocular condition affecting the quality of life of the patients. The personalized first-line treatment primarily relies on dynamically developing artificial tears, gels, eyelid sprays, and other therapeutic steps. Management also targets MGD and the ocular surface microbiome. The use of herbal and other natural compounds (honey, human tissues) in DED treatment is an emerging trend in ophthalmology due to their potential benefits and minimal side effects. In conclusion, natural pharmaceutical compounds offer promising adjunctive treatments, but healthcare professionals should further educate themselves on this hot topic, not only by choosing the most adequate product for their patients but also by being careful of not proven herbal compounds or herbal-pharmacological interactions. Interdisciplinary collaboration among healthcare professionals is essential to address the multifaceted nature of DED and optimize patient outcomes. Empathy, patient education, and psychological support are integral components of holistic care, fostering patient adherence and improving quality of life.
Acknowledgments. – The authors express their gratitude to Adrián Nagy for the digital graphical illustration. Fig. 1 is edited by Adrián Nagy.
Conflicts of interest. – The authors do not have conflicts of interest.
Funding. – This article received no external funding.
Authors contributions. – Conceptualization, writing, original draft preparation, T.R.; review and editing, T.R. and A.C. Both authors have read and agreed to the published version of the manuscript.