Introduction
Methotrexate (MTX) is a widely used anti-cancer drug. Its mechanism of action involves inhibiting the formation of tetrahydrofolate. As a result, it blocks the growth of tumour cells. MTX has been used effectively to treat a variety of cancers, such as head and neck cancer, graft-versus-host disease, and childhood acute leukaemia. However, the clinical use of MTX is limited due to the risk of severe side effects, including hepatotoxicity, low white blood cell counts, and ulcerative stomatitis. Given the therapeutic importance of MTX and the need to manage its side effects, the development of a sensitive, accurate, and rapid detection method is crucial for clinical applications and pharmacological research. Accurate monitoring of MTX levels can help clinicians optimize dosages, minimize adverse effects, and improve patient outcomes during MTX treatment [1-3]. Over time, researchers have utilized various analytical techniques to detect and quantify MTX concentrations. Some commonly used methods include spectrophotometry, high-performance liquid chromatography, capillary electrophoresis, chemiluminescence, and liquid chromatography tandem mass spectrometry [4-8].
Electrochemical techniques offer several advantages for the detection and quantification of target species [9-20]. These advantages include high sensitivity, good selectivity, rapid methodologies, portability, and economical and simple operation [21-26]. However, the direct electrooxidation of target species at bare electrode surfaces may not be suitable for analytical applications due to high overpotentials and slow electrode kinetics. To address this, researchers have developed and utilized various chemically modified electrodes (CMEs) [27-32]. The use of CMEs can significantly lower the overpotentials and increase the oxidation current response, thereby improving the analytical performance for the detection of target analytes [33-38].
The choice of the appropriate electrode modification material, which should possess good conductivity and electrochemical properties, is crucial for the development of highly precise and sensitive analytical methods for the effective detection of MTX and other target analytes. Nanotechnology has been recognized as one of the most important technologies in recent years, which has received much attention due to its wide applications in various fields [39-49]. Especially, nanostructures have attractive properties for electrochemical sensing applications [50-55].
Layered double hydroxides (LDHs) have emerged as promising two-dimensional host/guest nanomaterials with a wide range of applications, including adsorption, catalysis, supercapacitors, fire retardants, electrochemical sensors, and biochemical applications. LDHs are composed of divalent and trivalent metal ions, where the divalent metal ions (e.g., Mg2+) can be substituted by other divalent ions, such as Al, Ni, Mn, Co, or Cu, with a similar ionic radius. This versatility in metal composition allows for tailoring LDH properties to suit specific applications. The open and layered structure of LDHs, combined with their high biocompatibility, make them attractive materials for use as stable matrices or effective redox mediators in diverse electrochemical sensor applications. In these contexts, LDHs exhibit exceptional catalytic properties that can enhance the sensitivity and performance of electrochemical sensing platforms. The unique structural and chemical characteristics of LDHs, such as their tunable composition, high surface area, and ion-exchange capabilities, have contributed to their growing popularity in the development of advanced electrochemical sensors. Researchers have actively explored the integration of LDHs into sensor designs to leverage their unique properties and improve the overall analytical performance for the detection of various target analytes. The versatility and advantageous properties of LDHs have led to their increasing adoption in a wide range of electrochemical sensing applications, making them a valuable class of materials for the advancement of sensitive, selective, and robust analytical techniques [56-59].
Ionic liquids (ILs) are characterized by low melting points, often at or below ambient temperature. In recent years, ILs have gained significant attention as a frontier and novel area of research due to their unique chemical and physical properties. Some of the key characteristics of ILs include negligible vapor pressure, high conductivity, wide electrochemical windows, and high chemical and thermal stability. Recently, ILs have been proposed as efficient binders for the preparation of ionic liquid-based paste electrodes. The unique properties of ILs, make them attractive candidates for the development of advanced electrochemical sensing platforms. The use of ionic liquid-based paste electrodes has been explored in various analytical applications, taking advantage of the enhanced stability, conductivity, and electrochemical performance provided by the incorporation of ILs. This has led to the emergence of ILs as an innovative and promising class of materials in electrochemical sensors and electroanalytical techniques [60-63].
Based on the previous information provided, the current study focused on developing an advanced electrochemical sensor for the determination of MTX. In the first part of the study, the electrochemical behaviour and detection of MTX was investigated using a bare carbon paste electrode (CPE). However, the direct electrooxidation of MTX at the bare CPE surface exhibited some limitations, such as slow electrode kinetics and high overpotentials. To address these limitations, we focused on the preparation of the innovative electrochemical sensor by combining the advantageous properties of ILs and layered double hydroxides (LDHs). Specifically, an IL/Ni-Co-LDH-based sensor (IL/Ni-Co-LDH/CPE) was fabricated and its performance in the determination of MTX was evaluated. It is expected that the integration of IL and Ni-Co-LDH in the sensor design contributed to the enhanced electrochemical performance, leveraging the unique properties of these materials, such as high conductivity, catalytic activity, and efficient electron transfer kinetics.
Experimental
Reagents and chemicals
Graphite and liquid paraffin were purchased from Sigma Aldrich and Merck, respectively. Methotrexate (MTX) and other chemicals were of analytical grade and used without further purification. PBS was used as supporting electrolyte. All the electrochemical measurements and data collection were performed at room temperature (25 °C).
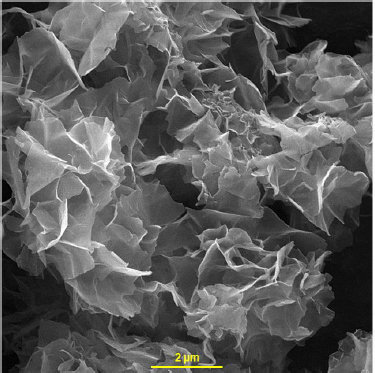
The synthesis and characterization of Ni-Co LDH nanosheets were described in our previous work [64].Figure 1 shows the FE-SEM image of Ni-Co LDH nanosheets.
Preparation of modified electrode
The bare CPE was prepared by thoroughly mixing graphite powder and liquid paraffin in a mortar and pestle. The composite mixture was then packed into the end of a glassy tube. Electrical contact was made by inserting a copper wire into the back of the composite mixture in the glassy tube.
The modified electrode, IL/Ni-Co-LDH/CPE, was prepared by mixing the unmodified CPE composite (graphite and liquid paraffin) with ionic liquid (IL) (1-butyl-3-methylimidazolium hexafluorophosphate) and nickel-cobalt layered double hydroxide (Ni-Co-LDH) using the same method as the bare CPE. The resulting IL/Ni-Co-LDH/CPE was then packed into the end of a glassy tube, and electrical contact was established by inserting a copper wire into the back of the composite.
Results and discussion
Electrochemical behavior of MTX
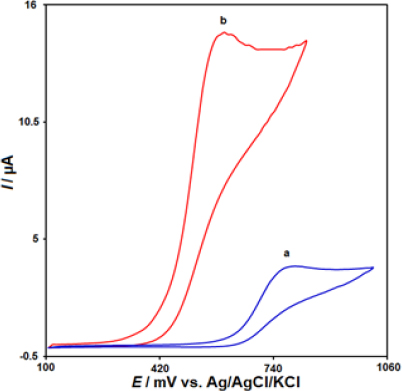
The electrochemical behaviour of MTX was investigated using cyclic voltammetry (CV) at different electrode surfaces in a 0.1 M PBS at pH 7.0, as shown inFigure 2. At the unmodified CPE, a small oxidation peak was observed with a peak current of 3.7 μA and a peak potential of 790 mV (curve a). In contrast, at the IL/Ni-Co-LDH modified electrode, the peak potential was negatively shifted to 600 mV, and the peak current was approximately 4 times higher than that of the bare CPE (curve b). This significant enhancement in the peak current was attributed to the combined effects of the electron acceleration and catalytic properties of the IL/Ni-Co-LDH/CPE modifiers, which acted synergistically to improve the electrochemical detection of MTX. The results clearly demonstrate that the IL/Ni-Co-LDH/CPE sensor exhibited excellent electrocatalytic performance towards the oxidation of MTX compared to the unmodified CPE. Therefore, all the remaining electrochemical studies were conducted using the IL/Ni-Co-LDH/CPE sensor to take advantage of its superior sensitivity for the determination of MTX.
Influence of scan rate
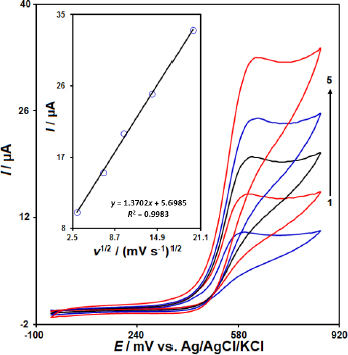
Figure 3 illustrates the impact of varying scan rates on the oxidation currents of MTX. Increasing the scan rate led to an enhancement of the peak currents. Moreover, the linear relationship between the Ip and the square root of the scan rate (ν1/2) suggests that the oxidation process is diffusion-controlled (Figure 3 inset). This finding is significant, as it provides insights into the mechanism of the electrochemical detection of MTX at the IL/Ni-Co-LDH/CPE sensor and supports the excellent electrocatalytic properties of the IL/Ni-Co-LDH/CPE towards the oxidation of MTX.
Chronoamperometry
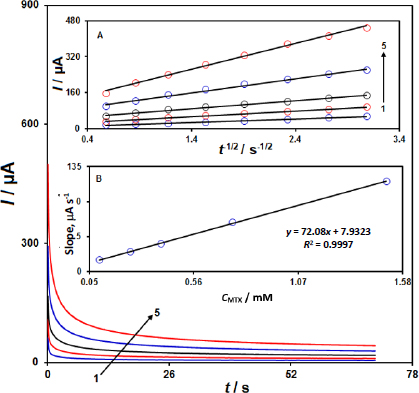
The diffusion coefficient (D) of MTX was determined using chronoamperometry experiments at the IL/Ni-Co-LDH/CPE sensor.Figure 4 shows the chronoamperograms recorded for different concentrations of MTX (0.1 to 1.5 mM) at a step potential of 650 mV. The slopes of the Cottrell plots (current vs. the inverse square root of time) for MTX, as shown inFigure 4A, were used to calculate the diffusion coefficient according to the Cottrell equation. Using the slope obtained from the plots and the Cottrell equation, the diffusion coefficient (D) of MTX was calculated to be 5.4×10-5 cm2/s.
This value of the diffusion coefficient provides important information about the mass transport characteristics of MTX at the IL/Ni-Co-LDH/CPE sensor, which is relevant for understanding the electrochemical behaviour and the sensor performance.
Electrochemical determination of MTX at IL/Ni-Co-LDH/CPE
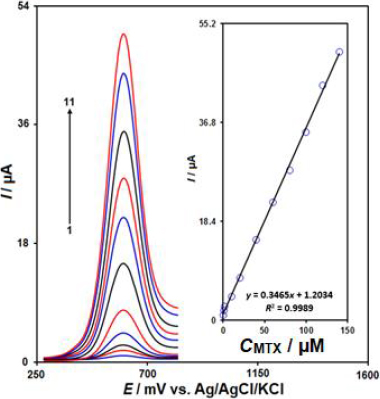
Figure 5 displays the differential pulse voltammetry (DPV) curves corresponding to the electrocatalytic oxidation of MTX at the IL/Ni-Co-LDH/CPE, recorded at a scan rate of 50 mV/s. As shown inFigure 5, the oxidation peak of MTX was observed at a potential of 600 mV. Upon increasing the concentrations of MTX in 0.1 M PBS (pH 7.0), the oxidation peak currents increased linearly. The inset inFigure 5 shows the calibration plot of the oxidation peak current versus the MTX concentrations. The oxidation current had a linear relationship with MTX concentrations ranging from 0.02 to 140.0 μM, (R2 = 0.9989). The LOD (3Sb/m) for MTX was calculated to be 0.006 μM. The sensitivity of the IL/Ni-Co-LDH/CPE sensor towards the electrochemical detection of MTX was found to be 0.3465 μA/μM. These results demonstrate the excellent analytical performance of the IL/Ni-Co-LDH/CPE sensor for the sensitive determination of MTX, which can be attributed to the synergistic effects of the ionic liquid and the Ni-Co-LDH modifiers on the electrochemical oxidation of MTX.
Determination of MTX in MTX tablets and urine samples
To assess the applicability of the proposed IL/Ni-Co-LDH/CPE sensor, it was used for the determination of MTX content in commercial pharmaceutical preparations and urine samples using standard addition method. The obtained results are given inTable 1. The recoveries demonstrate a very good accuracy of the proposed electrochemical method for the determination of MTX in both clinical preparations and biological fluids. These results indicate that the IL/Ni-Co-LDH/CPE sensor can be successfully applied for the sensitive and accurate determination of MTX in pharmaceutical formulations and clinical samples, with good reliability.
Conclusion
In this study, a novel electrochemical sensor for the detection of MTX was successfully developed using IL and Ni-Co-LDH as the modifiers. The Ni-Co-LDH material provided good properties, which enhanced the electrochemical performance of the sensor. The proposed IL/Ni-Co-LDH/CPE sensor exhibited a wide LDR of 0.02 to 140.0 μM and a remarkably low LOD of 0.006 μM for MTX. This study presents an innovative strategy for the sensitive electrochemical detection of MTX, which leverages the synergistic effects of IL and Ni-Co-LDH as the sensing platform. The remarkable analytical performance demonstrates the potential of combining these two functional materials in an electrochemical assay for the rapid and reliable quantification of MTX. The developed IL/Ni-Co-LDH/CPE sensor showed great promise for practical applications in the determination of MTX in clinical preparations and pharmaceutical formulations, with high sensitivity, accuracy, and reliability.