INTRODUCTION
Lipid oxidation and microbial growth are common quality defects in the food industry that affect not only consumer preference but also safety. To eliminate these quality defects, the use of food additives attracts the attention of the food industry (1,2). The quality parameters of food products are maintained by the addition of food additives (3). Synthetic food additives, which have antimicrobial and antioxidant effects, are widely used by the food industry. However, synthetic food additives such as nitrite, butylated hydroxyanisole and butylated hydroxytoluene are being questioned due to their toxicity and carcinogenic effects. The use of some of these food additives is restricted or prohibited by law because of their harmful effects on consumer health. Furthermore, the use of natural sources as food additives is increasing due to the expectations of consumers, legal agencies and the food industry regarding healthy food (4).
Ginseng (Panax ginseng C.A. Meyer) is a highly regarded therapeutical plant in the countries of East Asia. Besides its use as a traditional medicine for treatments of chronic metabolic syndromes, diabetes and cardiovascular disorders, ginseng also has antioxidant, anti-inflammatory, anticancer, antiobesity and antiviral properties. The pharmacological benefits of ginseng are attributed to phenolics, quinones, saponins, flavonoids, tannins, coumarins and alkaloids (5,6). Ginsenosides, such as saponin, are the main bioactive compounds of ginseng and responsible for its biological properties. Kwon et al. (7) have reported that the ginsenosides obtained from ginseng are divided into polar and less polar ginsenosides. The polarity of ginsenosides has been reported to determine the pharmacological activity, which decreases with the increasing degree of polarity (6).
The antioxidant activity of ginseng has been associated with the presence of biological molecules such as phenolic acids (8), polyacetylene (9), polysaccharide (10), saponin (11) and ginsenoside (12). Hussain et al. (13) stated that the importance of flavonoids for antioxidant activity should not be ignored. It has been found that ginseng increased the activity of antioxidant enzymes such as glutathione peroxidase and superoxide dismutase in rats (14). Guo et al. (10) stated that polysaccharides obtained from the stem of ginseng had higher antioxidant activity than those extracted from the root. Differences in harvesting and germination conditions of ginseng plants, different extraction methods and postextraction applications have been reported to have an effect on the antioxidant ability of ginseng extracts (15,16). Due to the large molecular mass of phenolic acids and ginsenosides in the structure of ginseng, some applications such as thermal application are used to increase its bioavailability. As a result of thermal application, maltol compounds with phenolic properties are formed (12). An increase in the nitric oxide binding activity and hydroxyl radical scavenging activity of phenolic compounds containing maltol has been reported, as well as a reduced rate of lipid oxidation due to the Fe3+ chelating activity of maltol itself (17).
Besides the antioxidant activity, ginsenosides obtained from ginseng are also responsible for the antibacterial and antifungal activities of ginseng (12). The antimicrobial activity of ginseng is explained by several mechanisms such as inhibition of microbial motility and quorum sensing, reduction of biofilm formation, disruption of cell wall structure and reduction of bacterial adhesion due to stimulation of the immune system (18).
The aim of this study is to evaluate the in vitro antioxidant activity of ginseng extract and to demonstrate its effects on the inhibition of lipid oxidation, chemical, microbiological and textural properties of cooked ground beef during 30 days of refrigerated storage.
MATERIALS AND METHODS
Materials
The roots of ginseng (Panax ginseng C.A. Meyer) plant were purchased locally (Tokay Herbs & Spices Store, Isparta, Turkey). The ginseng roots were chopped into small pieces and then ground into powder using a grinder (Arzum, Istanbul, Turkey). Ginseng powder was then stored at −80 °C. The 24-hour postmortem beef (Longissimus thoracis et lumborum) from approx. 1.5–2-year-old Simmental cattle was obtained from a local meat supplier, transferred to the laboratory under cold chain, ground, vacuum packaged and then kept in a freezer (−20 °C) until use.
Ginseng extraction
A mass of 20 g of ginseng powder was macerated for 2 days with 100 mL of ethanol (80 %; Merck, Darmstadt, Germany) in the dark. The ethanolic ginseng extract was filtered and the filtrate was held at 40 °C in a vacuum rotary evaporator (Hei-VAP; Heidolph Instruments, Schwabach, Germany) until all alcohol was removed (19).
Antioxidant capacity assays
FRAP anaylsis
FRAP was determined in the ginseng extract according to Ou et al. (20). The FRAP reagent was prepared by mixing 300 mmol acetate buffer (pH=3.6), 10 mmol 2,4,6-tripyridyl-s-triazine (TPTZ) (Merck, Darmstadt, Germany) in 40 mmol HCl (Merck, Darmstadt, Germany) and 20 mmol Fe(III) chloride (Merck, Darmstadt, Germany) (10:1:1). A volume of 100 µL of ginseng extract and 3 mL FRAP reagent were added and the absorbance was determined at 593 nm (T8+ UV/VIS spectrometer; PG Instruments Ltd., Leicestershire, UK). The results were reported as Fe(II) equivalents in mmol/g.
DPPH scavenging anaylsis
The scavenging activity of the ginseng extract was measured according to Dorman et al. (21). Different volumes of ginseng extract (20, 40, 60, 80 and 100 μL) were added to 600 µL of DPPH reagent (0.1 mmol) and total volume was completed to 6 mL with ethanol. Absorbance of the mixture was measured at 517 nm against a blank after 15 min of incubation in the dark at room temperature. Results were expressed as percentage of inhibition and IC50 value.
Anaylsis of total phenolic content
A volume of 1 mL of ginseng extract and 5 mL of 0.2 M Folin-Ciocalteu reagent (Merck, Darmstadt, Germany) were mixed for 3 min. A 7.5 % sodium carbonate (Merck, Darmstadt, Germany) was put into the mixture and then kept at room temperature for 30 min. To determine the TPC, the absorbance was measured at 720 nm. The results were reported in mg/g of gallic acid equivalents (22).
Meat sample preparation and design of experimental groups
Ground beef was thawed at 4 °C for 12 h. After adding 10 % pure water and 2 % NaCl, the thawed ground beef was divided into equal portions for the experimental groups. Experimental groups were formed according to the tested ginseng extract amounts (Table S1). After the addition of ginseng extract, the samples were filled in plastic centrifuge tubes with screw caps. For each experimental group, 50 g of ground beef was carefully filled into each of these tubes so that there was no air gap. In addition to experimental groups, an extra tube was filled to monitor the core temperature. After each filled tube had been put into a water bath set to 60 °C, the water bath temperature was increased to 85 °C. Then, the core temperature for the experimental groups was tracked with a thermocouple. The cooking process was terminated when the core temperature reached 74 °C. After removal of the cookout liquid, cooked samples were stored at 4 °C for 30 days.
Cooking loss analysis
The mass of the raw sample was recorded before cooking. After cooking, the liquid part was removed from the tubes that were cooled at room temperature and the mass was recorded again. Cooking loss was calculated according to the formula shown below.
where mr is the mass of the raw meat sample and mc is the mass of the cooked sample.
Physicochemical composition
Moisture, protein, fat and ash were determined according to AOAC methods (23-26). The pH was measured with a pH meter (Hanna Instruments, Leighton Buzzard, UK). After homogenization of 5 g sample in 50 mL distilled water, the pH was determined. Colour measurements were performed in triplicate from samples stored at 4 °C. CIE L*, a* and b* values were determined with Precise Color Reader TCR-200 (PCE Instruments, Southampton, UK) (27). The colour device was calibrated with the dark and white field standards before the measurements. Texture measurements were made by a texture analyzer (CT3; Brookfield, Middleboro, MA, USA) at room temperature. A 36 mm thick probe, 50 kg load cell, 0.5 cm thick sample, 0.35 mm penetration (70 % compression), 2 mm/s probe velocity before and after the test and 5 mm/s probe velocity during the test were implemented as analysis conditions. Hardness, cohesiveness, springiness, gumminess, chewiness, adhesiveness and resilience parameters were determined in meat samples.
Analysis of TBARS and LPO
Thiobarbituric acid reactive substances (TBARS) were analysed in accordance with the method described by Kilic and Richards (28) for monitoring the progress of lipid oxidation in meat samples. Propyl gallate and EDTA were added to the extraction solution of trichloroacetic acid (TCA) to avoid the formation of TBARS during the analysis. A mass of 2 g meat sample was stired in the extraction solution (12 mL). Meat samples were homogenized for 15 s and the homogenate was filtered through Whatman no. 1 filter paper. A volume of 1 mL of the obtained filtrate was taken and mixed with thiobarbituric acid (TBA) solution and then vortexed at 250×g. Then, the mixture was warmed up at 100 °C for 40 min. Then, the tubes were cooled in cold water. After cooling, the samples were centrifuged (Rotofix 32A; Hettich, Schwerin, Germany) at 2000×g for 10 min. Absorbance values were recorded at 532 nm versus a blank including TCA extraction solution (1 mL) and TBA solution (1 mL). TBARS concentrations were espressed as malondialdehyde in µmol/kg.
A method of detection of lipid hydroperoxide (LPO) described by Kılıç et al. (29) was used for the analysis of lipid hydroperoxide. Briefly, 1 g sample was homogenized for 30 s in 5 mL chloroform/methanol (1:1). After that, 3 mL NaCl (0.5 %) were added and vortexed for 30 s. Then, this mixture was subjected to centrifugation (Rotofix 32A; Hettich) for 10 min at 2000×g to achieve phase separation. After that, lower phase (2 mL) was taken and added to the 1.3 mL cold methanol/chloroform (1:1) mixture and vortexed. After adding 25 µL Fe(II) chloride (18 mM) and 25 µL ammonium thiocyanate (4.38 M), the samples were held at room temperature for 20 min and then their absorbance values were measured at 500 nm.
Microbiological analysis
To determine total aerobic mesophilic bacteria (TAMB), total coliform bacteria (TCB), yeast and mould (YM) counts, under aseptic conditions, 10 g meat samples were weighed into homogenizer bags and 90 mL of physiological saline were added. After homogenizing for 1 min, serial dilutions were prepared from this dilution and incubated at 30 °C for 48 h on a plate count agar (Merck, Darmstadt, Germany) for TAMB, at 37 °C for 48 h on eosin methylene blue agar (Merck) for TCB and at 25 °C for 72 h on potato dextrose agar (Merck) for YM. Colony counts were obtained at the end of the incubation and expressed as CFU/g (30).
Statistical analysis
The experiments were conducted in two replicates and the analyses were performed in three parallel experiments. The significant differences among the results were determined using the MiniTab® 19.1.1 package program (31). After applying the analysis of variance (one-way ANOVA) to the antioxidant capacity assays (DPPH, FRAP and TPC) of the ginseng extract and the post-production analyses (cooking loss, moisture, protein, fat and ash content, and texture analysis), the differences among the experimental groups were determined by Duncan’s multiple range test. As for pH, instrumental colour, TBARS, LPO and microbiological analysis, the statistical factorial design was six ginseng extract amounts (0, 0.1, 0.5, 1, 1.5 and 2 %) × six storage times (0, 3, 5, 7, 15 and 30 days) for cooked ground beef samples. The independent variables (ginseng extract dose and storage time) and replications were designed as fixed and random effects, respectively. The main effects and their interactions related to the independent variables were determined. The dependent variables were pH, instrumental colour, TBARS, LPO, total aerobic mesophilic bacteria, total coliform bacteria, and yeast and mould counts. In this model, the results were tested using the restricted maximum likelihood (REML) method with a confidence interval of 95 %.
RESULTS AND DISCUSION
The results of the antioxidant capacity assay
The results of the DPPH radical scavenging assay (data not shown) showed that IC50 and inhibiton percentage of the ginseng extract were (12.11±0.09) mg/mL and (70.2±0.8) %, respectively. Previous studies report on the binding activity of DPPH radical by the ginseng extract. Chung et al. (32) stated that DPPH values of ginseng extracted with methanol were between 18.08 and 25.61 %. Moreover, Lee et al. (33) reported 51.0 and 86.2 % DPPH radical binding activity in the ethanolic extract of red ginseng and puffed red ginseng, respectivelty. Ganguly et al. (34) stated that IC50 values of ginseng extract were 32.80 and 38.83 µg/mL in methanol and methanol/chloroform/water solvents, respectively. Jiang et al. (35) found the IC50 value of 12 mg/mL in the essential oil obtained from the leaves of ginseng. Zhao et al. (36) found that DPPH binding activity varied between 50 and 95 % and IC50 values were between 0.150 and 0.155 mg/mL in oligosaccharides obtained from the ginseng extract. Hussain et al. (13) also found that the inhibition value in the ginseng extract ranged from 53.12 to 62.84 %.
The total phenolic content (TPC) of ginseng extract, expressed as gallic acid equivalents (GAE) on dry mass basis, was (146.0±2.4) mg/g in our study. Lee et al. (33) found that the TPC, expressed as tannic acid equivalents, of ethanolic ginseng extract was 6.72 µg/g. Ganguly et al. (34) found that the total phenolic content, expressed as GAE, of ginseng extract in methanol and methanol/chloroform/water (1:1:1) was 97.38 and 109.65 µg/mg, respectively. Shahriar et al. (37) found that the TPC value, as GAE, after chloroform extraction of ginseng was 60.99 µg/mg. Pal et al. (38) reported that total phenolic content, as GAE, of ginseng extract in three solvents (methanol, chloroform and water) was 42, 66.72 and 88.58 µg/mg, respectively, and the reason for this difference in each solvent was explained by the polarity of the polyphenolic substances. Zhao et al. (36) found that the total phenolic content, as GAE, of four different oligosaccharides extracted from ginseng ranged from 1.91 to 3.51 µg/mg. Ryu et al. (39) stated that the breakdown of ginsenosides into large molecular structures and their conversion into small molecules increased the total phenolic content of ginsenosides approximately threefold.
In our study, the FRAP activity of ginseng extract, expressed as Fe2+, was (4.7±0.2) mmol/g. Lee et al. (33) found the FRAP activity, expressed as ascorbic acid equivalents, of ginseng extract in the range of 1.04–2.34 µg/g. Variation in the antioxidant activity of ginseng extract in previous studies is thought to be associated with the differences in the type and parts of ginseng plant used and the applied extraction conditions such as the type of extraction solvent, extraction time and temperature. All these factors greatly affect the content of antioxidants such as polyphenols, flavonoids and saponins in ginseng extract (40).
Effect of ginseng extract on the inhibition of lipid oxidation, chemical, microbiological and textural properties of cooked ground beef during refrigerated storage
Cooking loss results
The cooking loss results obtained in our study are shown inTable 1. The values for cooking loss were between 22.6 and 26.3 %. It was found that the addition of ginseng extract had a considerable effect on cooking loss (p<0.05). Although the groups containing ginseng extract had similar cooking loss values among themselves, they were higher than control (p<0.05). Our findings are supported by Kim et al. (41), who also found that the addition of ginseng to the pork sausage recipe resulted in an increase in cooking loss. An increased cooking loss due to the addition of ginseng associated with the change in meat pH was also reported (41). Similarly, in our study, the pH of the meat was found to decrease (Table 2) at the tested high ginseng extract amounts (G15 and G20).
Results are expresse as mean value±standard error. Values with different letters in superscript within a column are significantly different (p<0.05). G01 to G20=ginseng extract added to ground beef at 0.1, 0.5, 1.0, 1.5 and 2.0 %
Results are expressed as mean value±standard error. Values with different letters in superscript within each column are significantly different (p<0.05). G01 to G20=ginseng extract added to ground beef at 0.1, 0.5, 1.0, 1.5 and 2.0 %
The results of physicochemical composition analysis
Physicochemical composition of the experimental groups is shown inTable 1. The moisture content in the control was 69.0 %, while in the experimental groups containing ginseng extract it varied between 67.2 and 68.6 %. The results showed that groups containing ginseng extract were found to have similar moisture content to the control, which means that the addition of ginseng extract to the formulation did not affect the moisture content. Protein content determined in the experimental groups ranged from 27.3 to 29.7 % and did not reveal a significant difference between the groups with or without the ginseng extract. The amount of fat in the experimental groups varied between 3.5 and 3.9 % and it did not show any differences among the experimental groups either. The ash content of the experimental groups was determined in the range of 3.2–3.6 %. Although the ash content of the groups containing ginseng extract was similar to that of control, ash content of G10 group (3.6 %) was found to be higher (p<0.05) than that of G01 (3.2 %).
The results showed that the amount of ginseng extract and storage time had an effect on the pH values of the samples (p<0.0001), while their interaction (Table S1) was not a factor. Therefore, only the main effects (ginseng extract amount and storage time) and not their interaction will be discussed. In general, there was a decrease in pH values in the groups with ginseng extract of 1.5 % or more (p<0.05;Table 2). Regardless of the storage time, the pH values of the control (6.04±0.01) and groups G01 (6.07±0.01), G05 (6.06±0.01) and G10 (6.04±0.01) were similar. In addition, groups G15 (6.01±0.01) and G20 (6.01±0.01) were found to have similar pH values. Although the pH values of groups G15 and G20 were statistically lower than those of the other groups, these differences may not be significant in practical applications. Ibrahim et al. (42) also found that the use of ginseng extract in lamb patties resulted in lower pH values than the control. Regardless of ginseng extract, pH values increased (p<0.05) during the first 5 days of storage and then started to decrease (p<0.05) during the rest of the storage (day 0: 6.00±0.01, day 3: 6.08±0.01, day 5: 6.08±0.01, day 7: 6.01±0.01, day 15: 6.03±0.01 and day 30: 6.00±0.01). Ibrahim et al. (42) found that the increase in pH values during storage of lamb patties was due to ammonia produced by protein oxidation or degradation by proteolysis. It is also believed that the pH decrease observed after 5 days of storage is related to the activity of lactic acid bacteria. The decrease in pH of stored muscle foods may be related to the activity of lactic acid bacteria, which metabolise the carbohydrates in muscle food and convert them into lactic acid (43,44).
The effect of the amount of ginseng extract and storage time on the CIE colour values of the experimental groups is shown inTable 2. The analysis of variance revealed that the effect of the ginseng extract amount, storage time and their interaction on the CIE L*a*b* values was significant (p<0.0001). The lowest L* values on the day of processing (t=0) were obtained in group G20 (p<0.05). Although the L* values of groups G01, G05, G10 and G15 on the day of processing were similar to those of the control, the L* values of groups G10 and G15 were found to differ from each other (p<0.05). The results indicated that the L* values tended to increase (p<0.05) throughout storage, except for those of groups G05, G10 and G15, which were constant during the same period. After 30 days of storage, the lowest L* values were obtained in groups G15 and G20, while the highest L* values were found in the control and G01 (p<0.05). In general, the higher the amount of ginseng extract, the lower the L* value after 30 days of storage (p<0.05). There were no significant differences between the a* and b* values between the control group and the groups with the added ginseng extract on the day of processing. A decrease in a* values and an increase in b* values were observed in all experimental groups throughout the storage (p<0.05). After 30 days of storage, the a* values of the groups containing ginseng extract were similar to each other, but lower (p<0.05) than tose of the control. On the other hand, the highest (p<0.05) b* values were found in groups G01 and G05, while the other experimental groups had similar b* values. Kim et al. (41) found that the addition of ginseng increased the b* values of pork sausage, while the L* and a* values decreased. However, Cho et al. (45) claimed that the addition of ginseng powder did not affect the colour parameters of pork.
The results of the texture analysis of the experimental groups inTable 3 show no significant differences among the experimental groups in terms of hardness, adhesiveness, resilience, cohesiveness, springiness, gumminess and chewiness. Kim et al. (41) reported that the addition of ginseng reduced only the hardness parameters of pork sausage, but did not affect other parameters.
Results are expresses as mean value±standard error. Values with different letters in superscript in the same column are significantly different (p<0.05). G01 to G20=ginseng extract added to ground beef at 0.1, 0.5, 1.0, 1.5 and 2.0 %
TBARS and LPO values
The effect of ginseng extract amount and storage time on TBARS and lipid hydroperoxide (LPO) values of the experimental groups is shown inTable 4. The results indicate that TBARS values of all experimental groups containing ginseng extract were lower than of control on the day of processing (p<0.05). The TBARS values gradualy increased in all experimental groups throughout the storage (p<0.05). The interaction between the amount of ginseng extract and storage time showed that the TBARS values of control and groups G01 gradually increased during each storage day (p<0.05). In the meantime, TBARS values of G05 increased during the first 5 days of storage, remained stable during the period between days 5 and 7 and then increased again during the rest of the storage. Furthermore, TBARS values of groups G10, G15 and G20 remained constant during the storage between days 3 and 5, and then increased during the remaining storage time (p<0.05). After 30 days of storage, the highest TBARS values were obtained in the control and groups G01 and G05, while the lowest TBARS (26.79 % reduction) value was determined in group G20 (p<0.05). Generally, TBARS values decreased with increasing ginseng extract amount (p<0.05). Papuc et al. (46) pointed out that dried plants and essential oils successfully delay lipid oxidation in muscle food and that this effect is due to the fact that polyphenols are good electron and proton donors. It has also been found that bioactive components such as triterpenes and saponins in ginseng can prevent chain reactions that occur during lipid oxidation (47). Lipid oxidation in cooked muscle foods can be influenced by pH, which affects the activities of prooxidants, especially haem iron. The iron-catalyzed oxidation has been reported to decrease with an increase in pH from 2 to 10 and myogloblin-catalyzed oxidation decreased with an increase in pH (48). Since the differences in pH among the groups in the present study were negligible from a practical point of view, pH is thought to be insignificant factor modulating the development of lipid oxidation. In addition, Ibrahim et al. (42) found that ginseng extract had higher antioxidant activity than jojoba and ginger extracts, and the lowest TBARS values after 9 days of storage were found in the groups with added ginseng extract. In another previous study in which ginseng extract was added to the meat emulsion model, the researchers found that the use of 2.5 % ginseng extract prevented lipid oxidation of meat products (46). On the other hand, Cho et al. (45) found that the addition of ginseng powder could maintain TBARS content in pork chops stored at 4 °C for 15 days constant for up to 5 days, but lipid oxidation could not be prevented during the remaining storage time.
Results are expressed as mean value±standard error. For each tested parameter in the table, the values with different letters in superscript are significantly different (p<0.05). G01 to G20=ginseng extract added to ground beef at 0.1, 0.5, 1.0, 1.5 and 2.0 %
The results regarding the effects of ginseng extract amount and storage time on LPO values of the experimental groups showed that there were no significant differences among the experimental groups on the day of processing. It was found that the LPO values of all experimental groups increased progressively during storage (p<0.05). On the other hand, the LPO values of the groups containing 1 % or more ginseng extract increased during the first 3 days of storage, remained stable between day 3 and 7 and then showed an increasing trend again during the rest of the storage (p<0.05). After 30 days of storage, the highest LPO values were obtained in the control group, while the lowest LPO (52.25 % reduction) values were determined in group G20 (p<0.05). Generally, LPO values decreased with increasing ginseng extract amount, but the same trend was not observed in groups G01 and G05. The LPO values of G01 were lower than the LPO values of G05 (p<0.05).
The results of microbiological analysis
The results of the microbiological analysis (Table 5) showed that the total aerobic mesophilic bacteria (TAMB) in the raw meat material before heat treatment was 4.60 log CFU/g, while after cooking it was <1 log CFU/g in all experimental groups. After 30 days of storage, the TAMB in the control group was 3.4 log CFU/g, but in the other experimental groups containing ginseng extract, it was <1 log CFU/g. Ibrahim et al. (42) also pointed out that the use of ginseng extract reduced the load of aerobic mesophyll bacteria in lamb patties. While the TAMB in the experimental groups containing ginseng extract was below the detection limit in our study, Ibrahim et al. (42) reported the TAMB in the experimental group containing ginseng extract of 2.51 log CFU/g. An increasing trend in TAMB in the control group was observed during refrigerated storage. Furthermore, Kim et al. (41) found that the total aerobic plate count in pork sausage decreased with increaseing ginseng extract amount.
Values with different letters in superscript within the same column are significantly different (p<0.05). TAMB=total aerobic mesophilic bacteria, TCB=total coliform bacteria, YM=yeasts and moulds, CFU=colony forming units. G01 to G20=ginseng extract added to ground beef at 0.1, 0.5, 1.0, 1.5 and 2.0 %
The total coliform bacteria (TCB) was 3.69 log CFU/g in raw meat material (data not shown), but after heat treatment, it was <1 log CFU/g in all experimental groups (Table 5). In all experimental groups, TCB was also <1 log CFU/g during the entire storage period. On the other hand, the yeast and mould (YM) count in the raw meat material before heat treatment was 3.30 log CFU/g, but after heat treatment, it was <1 log CFU/g in all experimental groups. The YM count determined in all experimental groups was <1 log CFU/g until the end of storage. Ibrahim et al. (42) found that ginseng extract had a higher influence on TAMB than YM count in lamb patties. Overall, the inhibited microbial growth in the experimental groups containing ginseng extract could be due to the antimicrobial activity of the components of ginseng extract used in our study. The components of ginseng extract, such as ginsenosides, have been reported to interact with microorganisms and prevent microbial growth by inhibiting microbial motility and quorum sensing, reducing biofilm formation, disrupting cell wall structure and reducing bacterial adhesion due to stimulation of the immune system (18).
CONCLUSIONS
The in vitro results on antioxidant capacity showed that the ginseng extract had iron ion reducing and free radical scavenging activities. The results showed that the addition of ginseng extract caused a decrease in pH and an increase in cooking loss in ground beef. Furthermore, the addition of ginseng extract caused a decrease in brightness and redness values, but an increase in yellowness values in cooked ground beef. Ginseng extract did not affect the texture parameters and the proximate composition of cooked ground beef. The used ginseng extract showed the ability to inhibit lipid oxidation in cooked ground beef, and this effect increased with increasing amount of ginseng extract. The results indicated that aerobic mesophilic bacteria were more inhibited at the end of storage in cooked ground beef with ginseng extract than in those prepared without it. The growth of yeasts, moulds and coliform bacteria was not observed in any of the experimental groups during 30 days of storage, regardless of the addition of ginseng extract. In conclusion, the study results show that ginseng extract can be used as a natural preservative to ensure oxidative and microbial stability in the ready-to-eat meat products. Further research is needed to determine the effects of ginseng extract on the sensory properties of the meat products, to ensure that the food meets consumer demands.

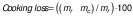 /1/
/1/