1. Introduction
Food and Agriculture Organization has proposed a NOVA food classification system that divides foods into four groups based on the type, extent, and purpose of industrial processing: unprocessed or minimally processed foods (UMPFs), processed culinary ingredients (PCIs), processed foods (PFs) and ultra-processed foods (UPFs) (Monteiro et al., 2019). One of the greatest concern today is the increasing consumption of UPFs, since it is suggested that this leads to an unbalanced diet (Monteiro et al., 2019; Elizabeth et al., 2020; Marino et al., 2021). The group of UPFs includes formulations of ingredients, mostly industrially produced, in the form of ready-to-eat products, prepared and ready-to-heat products. In addition, it includes sport foods, supplements and products to which nutrients and phytochemicals have been added for fortification (Monteiro et al., 2019).
Considering the fact that UPFs are more available and their consumption is possible at any time and regardless of the place (Monteiro et al., 2013; Monteiro et al., 2019), they are attractive for the consumption by different population groups in the life span (Marino et al., 2021; Lauria et al., 2021; de Miranda et al., 2021a; Mertens et al., 2022). In several European countries, between 14% and 44% of adults' daily energy intake comes from UPFs, and in countries where adults consume a higher proportion of energy from UPFs, the burden of overweight and obesity is higher (Mertens et al., 2022). In addition, there is evidence in the available literature that the consumption of UPFs may be positively associated with the prevalence of cardiovascular disease and other non-communicable and metabolic diseases (Monteiro et al., 2019; Elizabeth et al., 2020; de Miranda et al., 2021b). Moreover, higher consumption of UPFs may reduce diversity and affect the composition of the gut microbiota (Cuevas-Sierra et al., 2021; Fernandes et al., 2022), as they often contain large amounts of added sugars, fat, and salt, and they lack fibre and other plant-based nutrients. This can subsequently lead to an imbalance in the production of postbiotics, especially short-chain fatty acids (Garcia-Mantrana et al., 2018). However, there is also a criticism of this system of food classification and the theory that increased consumption of UPFs is the cause of the occurrence of diseases. It is emphasized that it is necessary to evaluate the impact of consumption of UPFs on diseases in terms of the overall dietary pattern (Marino et al., 2021).
Athletes, among others, could be a population group that reaches for ultra-processed foods more often. However, there is no study in the available literature that evaluates the consumption of processed foods in athletes. Namely, due to the lack of time between training sessions or competitions, athletes consume available foods that help them meet their daily nutritional needs and promote recovery (Calleja- González et al., 2016; Thomas et al., 2016). In addition, athletes can also eat more pack snacks or food and mini-meals when travelling (Esen et al., 2022). These foods often include ready-to eat snacks, sport drinks, sport bars, sport gels, sport confectionery, protein supplements, foods fortified with macronutrients, vitamins, minerals and other substances such as creatine, caffeine, nitrites and β-alanine, etc. (Sousa et al., 2015; Calleja- González et al., 2016; Thomas et al., 2016; Esen et al., 2022). This type of food belongs to the group of UPFs (Monteiro et al., 2019). The impact of nutrition on athletic performance is well known (Thomas et al., 2016; Holway and Spriet, 2011). During the competitive season, athletes try to avoid high-energy and nutrient-poor foods, most of which are found in the PFs and UPFs group, as this can impair their health, body composition and performance (Eck and Byrd-Bredbenner, 2021; Nishisaka et al., 2022), especially if they seek professional nutritional advice (Tsoufi et al., 2017). On the other hand, athletes have been shown to reach for these foods during training or post-season, which can subsequently lead to a change in body composition and cause a drop in athletic performance (Eck and Byrd-Bredbenner, 2021; Nishisaka et al., 2022; Tsoufi et al., 2017). In addition, the consumption of high-energy and nutrient-poor foods can alter the gut microbiota of athletes, which can affect their health, physical performance, and exercise capacity (Bongiovanni et al., 2021; Jäger et al., 2019; Marttinen et al., 2020).
In view of this, the objective of this study was to determine the proportion of processed foods in the diet of elite basketball players and to observe what type of processed foods they consumed the most. In addition, to determine whether there is a difference in anthropometric characteristics, neuromuscular and cardiovascular fitness, and gut microbiota between basketball players in relation to the proportion of daily energy from processed foods.
2. Materials and methods
Study design and participants
This cross-sectional observational study was conducted in March 2021. The study presents the results of the influence of basketball players' dietary habits on their anthropometric characteristics, neuromuscular and cardiovascular fitness, and gut microbiota at the baseline of the study "Effects of Soluble Dietary Fiber on Sport Efficiency and Fatigue Delay in Top Basketball Players", which was a part of the research project "Exploring Gut Microbiome Equilibrium" (Grant HRZZ_IP_06_2016_3509) funded by the Croatian Science Foundation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Faculty of Medicine, University of Zagreb (641-01/21-02/01). In addition, the study was registered with ClinicalTrials.gov under identifier NCT05726435.
Young male professional basketball players from one team (Zagreb, Croatia) were allocated in the study. Players who had taken antibiotics in the last 3 months, players with type 1 and type 2 diabetes, and players who had taken ergogenic agents in the last 30 days before the start of the study were excluded. In addition, players who had taken probiotics 3 months before the study and during the study were excluded. Of the total of 20 basketball players, 1 participant was excluded, and 2 declined to participate. Out of 20 initial players that have given written informed consent to participate in the study, 17 (85%) players have passed these inclusion criteria.
The study was conducted during the competitive season. All players had the same training process without the possibility of additional training that could affect the results. During each week they had two training sessions per day, and on the weekends they had a game and a day off. No additional friendly matches were played during the study due to the epidemiological measures of the COVID pandemic at the time. During the period of data collection, not a single included player was injured or sick.
Data collection extended over a total period of 2 weeks. Nutritional data were collected during the first 10 days, followed by anthropometric measurements and tests of explosive power, and aerobic and anaerobic capacities over the next 3 days. On the last day of data collection, players brought a stool sample for gut microbiota analysis.
Dietary assessment
Dietary intake data were collected using dietary records for 3 consecutive days (2 weekdays and 1 weekend day). All players received detailed instructions on keeping dietary records and how to measure their food intake. All food and beverages consumed had to be weighed or reported in household measurements, if there was no way to weigh the food. Data from the dietary records were used to estimate players' energy and macronutrient intake, as well as the proportion of differently processed foods in their diets. Results were expressed as the mean values of 3 days of consumption.
Energy and macronutrient intake was analyzed using “Prehrana” software (In-fosistem d.d, Zagreb, Croatia) based on Croatian food composition tables (Kaić Rak and Antonić, 1990). For products that were not included in the food composition tables, the software was supplemented with the energy and nutrient content from the nutritional information on the product labels.
All foods and beverages reported in the dietary records were divided into subgroups within the four groups according to the NOVA food classification system (Monteiro et al., 2019). The unprocessed and minimally processed foods group (UMPFs) consisted of eleven food subgroups that included natural edible parts of plants and animals, eggs, fungi, algae, drinking water, fresh or pasteurized milk, yogurt, and other minimally processed foods. The processed culinary ingredients (PCIs) group was divided into four subgroups that included salt, vegetable oils, lard, butter, sugar, molasses, honey and starch. The processed foods group (PFs) consisted of five subgroups and included products made by adding ingredients from the 2nd group, as well as products pre-served by non-alcoholic fermentation, canning and bottling. Eleven subgroups belonged to the ultra-processed foods (UPFs) group, which included formulations of ingredients that are mostly industrial, produced by a range of industrial processes. This group includes ready-to-eat products, preprepared and ready-to-heat products. The relative energy intake of the groups and subgroups was calculated as a percentage of the total daily energy intake for each player.
Anthropometric measuremnets
Anthropometric measurements were performed according to standard protocols by experienced trained people and included measurements of body weight, height, and skinfold thickness. Body weight was measured on the octopolar scale (OMRON BF - 511) with an accuracy of ± 0.1 kg, and body height was measured on the anthropometer (Siber-Hegner GPM anthropometer) with an accuracy of ± 0.1 cm. Skinfold thickness at seven sites (arm skinfold, back skinfold, chest skinfold, abdominal skinfold, suprailiac skinfold, thigh skinfold, axillary skinfold) on the right side of the body was measured with a caliper (Harpenden, British Indicators, West Sussex, UK) with an accuracy of ± 0.2 mm. Skinfold thickness was measured three times, and the median of the measurements was included in further data analysis. Body mass index (kg/m2) was used to assess weight status and was calculated from body height and body weight data. The percentage of body fat was calculated according to the equation in which body density was calculated from the sum of the seven skinfold thicknesses (Brožek et al., 2006; Jackson and Pollock, 1985).
Neuromuscular and cardiovascular fitness
In evaluating players’ performance, emphasis was placed on lower extremity explosive strength (concentric and eccentric contractions). The following tests were per-formed to evaluate sport-specific performance: explosive power of the vertical type (SJ - squat jump, CMJ - countermovement jump, CMJLF and CMJRF - countermovement jump LF - left foot and RF right foot, VJMAX - maximum countermovement jump, RJ15S - repeated countermovement jumps 15 s, and RSJ5 - five repeated stiffness jumps) using the Optojump system (Microgate, Bolzano, Italy) and explosive power of the sprint type (5 m sprint - EP05M Explosive Power 5 m, 10 m sprint - EP10M Ex-plosive Power10 m, and 20 m sprint - EP20M Explosive Power 20 m) using the Power-timerNewtest system (Oulu, Finland). Cardiovascular endurance was assessed with cardiovascular endurance tests (BEEP test) and anaerobic endurance tests 300MRT (300-meter test (15x20) with PowertimerNewtest (Oulu, Finland) (Paradisis et al., 2014).
Gut microbiota
Fecal samples were collected from players and stored at -80 °C using QIAamp® PowerFecal® DNA Kit, in accordance with the manufacturer’s instructions. The microbiome collective DNA was isolated from players’ fecal samples. DNA concentration was quantified using Promega Quantus Flourometer and sent to amplicon sequencing of variable regions 3 and 4 of the 16S rRNA genes (341f/806r primer set) at Molecular Research Lab (MRDNA, Texas, USA). Raw data was downloaded from Illumina’s BaseSpace Sequence Hub in the form of .fastq files and processed using QIIME2 version 2020.6 (Bolyen et al., 2019), following the “Moving pictures'' tutorial. Statistical significance of difference in microbial abundance was assessed using STAMP v 2.1.3. software (Parks et al., 2014).
Statistical analysis
Data were analyzed descriptively and statistically using IBM SPSS Statistics v. 23.0, release 2015 (IBM SPSS Statistics for Windows, version 23.0. Armonk, NY, USA: IBM Corp.). Continuous data were expressed as mean and standard deviation or median and interquartile range for normally distributed and non-normally distributed variables, respectively. The distribution of the data was tested using the Shapiro-Wilk normality test. For further analyses, all participating players were divided into two clusters according to the proportion of NOVA food groups (UMPFs, PCIs, PFs, UPFs) in total daily energy intake using K-Means cluster analysis, one cluster with higher and one cluster with lower intake of a particular food group. Two-tailed Welch's t-test was used to detect significant differences in anthropometric characteristics, neuromuscular and cardiovascular fitness, and microbial abundance between the two clusters. For all analyses, a significance level of p < 0.05 was used.
3. Results
The participants were young male professional basketball players aged 18.4 ± 0.7 years. Players had an average energy intake of 3274 kcal (2669 kcal – 3926 kcal), with the largest proportion of daily energy intake coming from fats (41.4%; 36.7% – 47.3%), followed by carbohydrates (38.7%; 34.9% – 43.2%) and proteins (16.6%; 14.7% – 21.9%). In terms of critical macronutrients, it should be noted that players had on average daily intake of 1.7 g/kg (1.2 g/kg – 2.1 g/kg) of proteins, 4.1 g/kg (2.2 g/kg – 5.2 g/kg) of carbohydrates, and 27.7 g (20.2 g – 37.3 g) of dietary fibres.
The results presented in Table 1 show that the most of the daily energy intake came from UMPFs (43.2% kcal), followed by UPFs (30.6% kcal), PFs (16.0% kcal), and PCIs (13.8% kcal). Within the UMPFs, the main energy sources were meat, poultry and eggs subgroups. PCIs contained mostly vegetable oil, while PFs were dominated by the fresh bread subgroup. The top two subgroups that contributed calorically to UPFs were biscuits, cakes, sweet bakery goods and reconstituted meat products. According to the daily energy contribution from NOVA food groups, players were divided into two clusters (Figure 1). Cluster 1 (35% of players) included the players who had a significantly greater contribution to daily energy intake from UPFs (49.1% kcal vs. 25.1% kcal; p > 0.001) and PFs (23.2% kcal vs. 13.8% kcal; p = 0.037), whereas players in cluster 2 (65% of players) had a significantly greater contribution to daily energy intake from UMPFs (47.2% kcal vs. 19.2% kcal; p > 0.001).
Table 1. Average proportion of daily absolute and relative energy intake according to NOVA food groups and subgroups in basketball players (n=17).1
1 All of the variables are presented as median (interquartile range). 2 Including tea, seeds, dried herbs and spices, spring and tap water. 3 Including salt, vinegar, sour cream, unsweetened cocoa powder. 4 Including beer, wine, peanut and peanut butter, gnocchi, tortilla. 5 Including bread-crumbs, chips, oat drink. 6 Players did not consume specific subgroups.
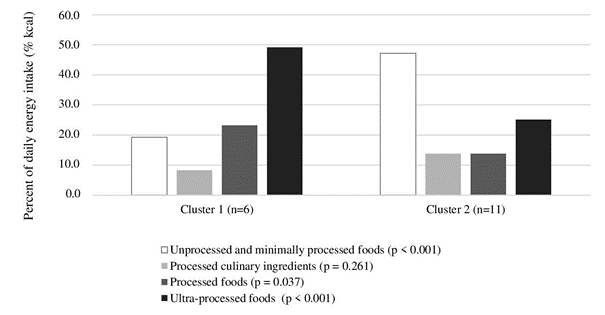
Figure 1. Average proportion of daily energy intake according to NOVA food groups in basketball players divided in two clusters. All of the variables are presented as median. Basketball players were divided in cluster with K-mean cluster analysis. Group differences are tested using two-sided Welch’s t-test (p < 0.05).
The results presented in Table 2. show no differences between the two clusters of players in anthropometric characteristics, neuromuscular and cardiovascular fitness. Figures 2-4 show the differences in relative abundance of taxa contributing to gut microbiota, at the order, family, and genus levels, respectively. Taxonomic level of order was the highest taxonomic category where statistically significant differences in abundance of taxa were detected. Order Veillonellales-Selenomonadales had higher abundance in the cluster with lower intake of UPFs (Cluster 2). Significant differences were also observed on lower taxonomic categories such as family Veillonellaceae (p = 0.040), and genus Agathobacter (p = 0.025) that also displayed higher abundance in the cluster with lower intake of UPFs (Cluster 2).
Table 2. Anthropometric characteristics, neuromuscular and cardiovascular fitness of basketball players.1
1 All of the variables are presented as mean ± standard deviation. 2 Sum of upper arm skinfold, back skinfold, chest skinfold, abdomen skinfold, suprailiac skin-fold, thigh skinfold and axillary skinfold. 3 Group differences are tested using two-sided Welch’s t-test (p < 0.05).
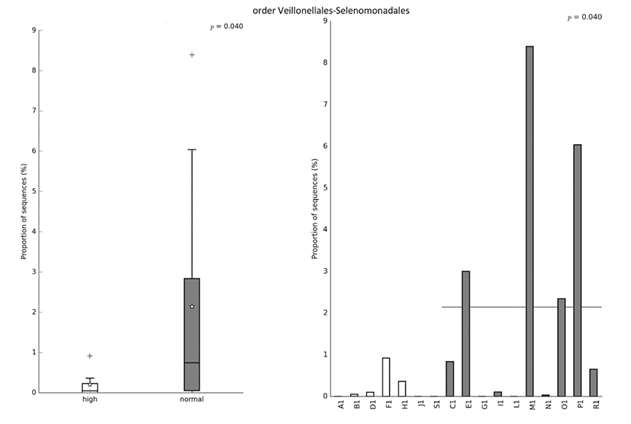
Figure 2. Significant difference in relative abundance at the order level between clusters based on the intake of NOVA food groups. Left image shows relative abundance of taxa for group while right image shows relative abundance of taxa for each subject. Statistical Analysis of Taxonomic and Functional Profiles (STAMP v2.1.3) was used to detect differentially abundant taxa at all taxonomy levels. Significant difference between groups was tested using the nonparametric White’s t-test with p-values < 0.05 considered significant.
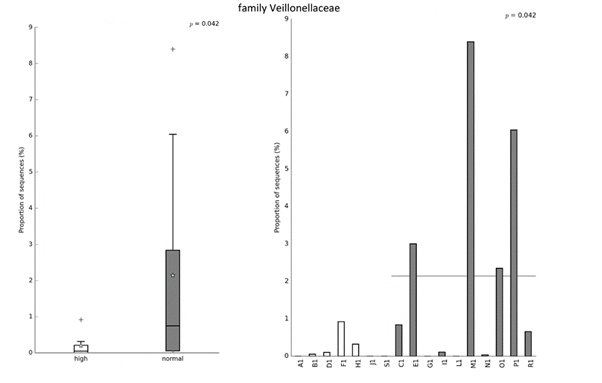
Figure 3. Significant difference in relative abundance at the family level between clusters based on the intake of NOVA food groups. Left image shows relative abundance of taxa for group while right image shows relative abundance of taxa for each subject. Statistical Analysis of Taxonomic and Functional Profiles (STAMP v2.1.3) was used to detect differentially abundant taxa at all taxonomy levels. Significant difference between groups was tested using the nonparametric White’s t-test with p-values < 0.05 considered significant.
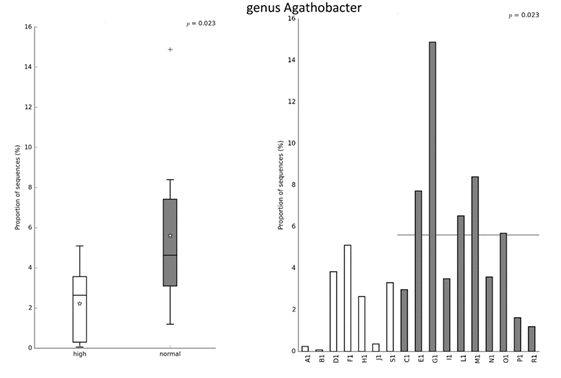
Figure 4. Significant difference in relative abundance at the genus level between clusters based on the intake of NOVA food groups. Left image shows relative abundance of taxa for group while right image shows relative abundance of taxa for each subject. Statistical Analysis of Taxonomic and Functional Profiles (STAMP v2.1.3) was used to detect differentially abundant taxa at all taxonomy levels. Significant difference between groups was tested using the nonparametric White’s t-test with p-values < 0.05 considered significant.
4. Discussion
To our knowledge, this is the first study to present data on processed foods consumption among elite basketball players during their competitive season. The results revealed that most of the daily energy intake of elite basketball players came from UMPFs. However, almost one third of energy came from UPFs. In addition, according to the cluster analysis, the majority of athletes (65%) relied mainly on UMPFs as their preferred sources of energy.
The most important determinants of athletes' food choices are performance and health, followed by sport, culture, nationality, sex and food environment (Pelly et al., 2022). Recently, it was suggested that athletes frequently consume lean proteins, eggs, fruit, vegetables, and Greek yogurt, because they believe that these types of foods have potential benefits for their health and performance (Eck and Byrd-Bredbenner, 2021). Indeed, in the present study, the basketball players had the largest proportion of daily energy intake from meat and poultry, vegetables, eggs, cereals and flour, and fruits and vegetables subgroups from UMPFs. In addition, it should be noted that in Croatian culture, the consumption of “cooked meals” still prevails over the consumption of “fast food” concepts (Čačić Kenjerić and Sokolić, 2021). Athletes often avoid fast food, fried foods, processed foods, and sugary drinks in competitive season because they find them unhealthy and eating them makes them sluggish (Eck and Byrd-Bredbenner, 2021; Tsoufi et al., 2017). These foods are mostly UPFs, and the basketball players from the present study consumed them in a smaller proportion of their daily energy intake, with the exception of the biscuits, cakes and sweet baked goods subgroup. In addition, the basketball players from the present study had a significant proportion of energy intake from vegetable oils, cheese, margarine, and meat - the food subgroups that are sources of fat in the diet. In fact, basketball players had a high percentage of daily energy intake from fat, which was above the recommendations. Proteins lead sports nutrition sales worldwide and are also popular with mainstream consumers (Arenas-Jal et al., 2020). However, the foods fortified with proteins and protein supplements did not greatly contribute to daily energy in take, and only 6 out of 17 players included in this study consumed this food subgroup. The player’s consumption of processed food was reflected in their energy and macro nutrients intakes, which are briefly presented in the study results. The basketball players had a mean daily energy intake of 3274 kcal which is in line with Greek elite basketball players during competitive season, but lower than that of a comparable group of Spanish players (Hassapidou et al, 2003; Schröder et al., 2004). However, based on this, we cannot conclude whether this was an adequate energy intake given their needs, as we have not estimated their daily energy expenditure. In addition, a large portion of the daily energy intake of the basketball players in the present study came from fat, which exceeds the official recommendations (Thomas et al., 2016; Holway et al., 2011). Since 2.5% of daily energy came from grains and flour subgroup, 2.6% from fruit, and 0.8% from vegetables and 6.3% from fresh bread, it is not surprising that the basketball players in the present study had a lower carbohydrate intake than that considered recommended. The basketball players only reached the recommendations for protein and dietary fibre intake (Esen et al., 2022; Thomas et al., 2016; Holway et al., 2011). This unbalanced diet may affect performance and body composition, especially in the competitive season in which the study was conducted (Thomas et al., 2016; Holway et al., 2011). In the present study, we did not observe the determinants of food choices of basketball players. However, it must be emphasized that the athletes did not eat meals organized by nutritionists after training or competitions. Moreover, they did not have a team nutritionist, so they most probably relied on individual advice. Accordingly, it is necessary to conduct further research on the determinants that influence the food choices of basketball players from Croatian culture in order to educate them and organize a system that will provide them the best nutritional support.
The second aim of the present study was to determine whether there is a difference in anthropometric characteristics, neuromuscular and cardiovascular fitness, and gut microbiota composition between basketball players in relation to the proportion of daily energy from processed foods. Since we do not yet have insight into the consumption of processed foods by basketball players from the available literature, we assumed two possible scenarios. The first is that basketball players who have an increased consumption of PFs and UPFs groups, focusing on high energy and low nutrient subgroups, will have a higher percentage of adipose tissue and poorer athletic performance (Monteiro et al., 2019; Elizabeth et al., 2020; Marino et al., 2021). Alternate hypothesis is that athletes who consume more PFs and UPFs groups, focusing on subgroups that include supplements or fortified foods, will have lower adipose tissue and better performance (Esen et al., 2022; Thomas et al., 2016; Holway et al., 2011; Eck and Byrd-Bredbenner, 2021; Tsoufi et al., 2017). However, we found no differences in anthropometric characteristics and neuromuscular and cardiovascular fitness between basketball players with higher and lower intakes of these PFs and UPFs groups. This could be due to the fact that basketball players did not consume any of the above food subgroups in a sufficiently large quantity to affect their anthropometrics. It should be taken into consideration that athletes included in the present study had high activity levels, unlike the non-athlete adult population, where increased intake of PFs and UPFs may have more pronounced negative health effects (Monteiro et al., 2019; Elizabeth et al., 2020; Marino et al., 2021). In addition, the present study was conducted during the competitive season and it is known that players have better eating habits during the competitive season than during the off-season (Eck and Byrd-Bredbenner, 2021; Tsoufi et al., 2017). It is also necessary to point out that the basketball players in this study did not differ from basketball players of their age in other leagues in European countries in terms of anthropometric measurements and neuromuscular and cardiovascular fitness (Boone and Bourgios, 2013; Gryko et al., 2018; Köklü et al., 2011; Tomovic et al., 2016; Vaquera et al., 2015). Therefore, future studies on this topic are warranted, especially with a larger sample of basketball players and other athletes, in multiple seasons, and with different measurements reflecting overall athletic performance.
Lastly, we would like to highlight the influence of consumption of processed foods on the gut microbiota of basketball players. Previously, it has been suggested that increased consumption of UPFs may affect the diversity and composition of the microbiota, leading to lower production of short-chain fatty acids, which have proinflammatory properties (Cuevas-Sierra et al., 2021). In athletes, it seems that gut microbiota may affect the production of bioactive metabolites, maintain of intestinal barrier function, reduce gastrointestinal illness, reduce respiratory illness, modulate immune system, improve energy metabolism, regulate the muscle metabolism, improve post-exercise recovery and mood-related outcomes (Marttinen et al., 2020). The analysis of the gut microbiota of basketball players in the present study showed that there was a statistically significant difference in the distribution of Veillonellales-Selenomonadales bacterial order in players with a lower intake of PFs and UPFs. More specifically, a difference was found between clusters of basket-ball players at the level of the family Veillonellaceae, order Veillonellales. In addition, the results of the present study showed a statistically higher prevalence of the genus Agathobacter in the cluster of basketball players with a lower consumption of PFs and UPFs. A higher occurrence of these bacteria considered as probiotics was found in the group of marathon runners and skiers, compared to that of the control group of people with a sedentary lifestyle (Kulecka et al., 2020). In addition, the presence of Agathobacter is positively related to the dietary fibre intake, and the players from the present study had adequate daily intake of dietary fibre. Veillonellales and Agathobacter are known to produce short-chain fatty acids, which are considered beneficial for athletic performance (Bongiovanni et al., 2021; Jäger et al., 2019).
With the aim of achieving homogeneity of the group in terms of neuromuscular and cardiovascular fitness and gut microbiota, a small group of elite basketball players was selected, which unfortunately implies some caution in the interpretation of the results. However, the study was conducted under controlled conditions and all basketball players had the same training process to prevent differences in the neuromuscular and cardiovascular fitness and the gut microbiota. The analysis of the stool samples by the research team occurred as soon as possible after each basketball player's stool sample had been collected, in order to avoid the risk of improper storage and transport. One of the limitations was the lack of measurement of the nature of each player's daily tasks, which could affect the quality of performance. We tried to prevent this by advising players not to change their lifestyle and daily commitments in the week before the measurement. Finally, the 3-day dietary record method was used to observe the consumption of food and beverages which can be tiring for athletes due to daily commitments and leads to a lower quality of records. However, the research team was able to discuss any ambiguities regarding food and beverage consumption from the dietary records with the players on an individual basis.
5. Conclusion
Basketball players have the most of their daily energy intake coming from UMPFs (43.2%) during the competitive season, with UPFs accounting for nearly one-third of daily energy intake. Majority of basketball players consumed more UMPFs than UPFs in the competitive season. The higher proportion of daily energy intake from UPFs did not affect anthropometric characteristics, and neuromuscular and cardiovascular fitness, but did alter the gut microbiota toward a reduction in portion of gut bacteria that may contribute to the formation of short-chain fatty acids. This study has raised the issue of processed food consumption on the performance of athletes. However, further studies are needed to determine what amount and what types of processed foods and by what mechanisms could negatively affect performance in different seasons and sports in order to establish better dietary guidelines for athletes.
Author Contributions: Conceptualization, A.S. and I.R.; methodology, I.R., J.Ž., A.S. and A.I.; formal analysis, J.Ž. and A.I.; investigation, E.H., J.Ž., A.S. and D.N.; data curation, J.Ž., A.S. and A.I.; writing—original draft preparation, E.H., I.R., J.Ž., A.S. and A.I.; writing—review and editing, A.I. and I.R.; visualization, J.Ž. and A.I.; supervision, I.R., A.S. and D.N.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding: This study was supported by the Croatian Science Foundation under the research project "Exploring Gut Microbiome Equilibrium" (Grant HRZZ_IP_06_2016_3509).
Acknowledgments: We would like to thank the basketball players and their coaches for their cooperation and support in collecting data for this study.
Conflicts of Interest: The authors declare no conflict of interest.
