1. Introduction
Plants that grow in saline environments are known as halophytes and account for only about 1% of known plant species (Yuan et al., 2019; Rozema et al., 2008). Because they have specific mechanisms for salt tolerance, they thrive in saline conditions that glycophytes and crops cannot tolerate (Aslam et al., 2011). Plants are considered halophytes if they can grow and reproduce in soils with a salt concentration higher than 200 mM, and some of them can thrive in high salinity soils even with salt concentration higher than in seawater (500 mM) (Yuan et al., 2019; Song and Wang, 2015; Flowers et al., 2015; Santos et al., 2015; Shabala et al., 2014; Yuan et al., 2013; Flowers and Colmer, 2008).
Halophytes can be classified in various ways, but the most common are classifications based on general ecological behaviour and distribution, plant growth response to salinity, and their salt uptake (Aslam et al., 2011; Waisel, 1978). These plants have different requirements and tolerances concerning their salt uptake, which is why they are categorised as obligate, facultative, and habitat-indifferent halophytes (Aslam et al., 2011; Sabovljević and Sabovljević, 2007). Obligate halophytes are also called true halophytes because they require salt for their normal development and growth. Facultative halophytes can grow under salt stress but also thrive well in environments without salt or with low salt concentrations. Habitat-independent halophytes normally grow in salt-free soils, but they thrive better than sensitive species in saline conditions. Based on their habitat, halophytes can be divided into hydro-halophytes (grow in aquatic soils or wet conditions) and xero-halophytes (grow in soils with high salinity and low water content) (Aslam et al., 2011). Halophytes can also be divided into three different categories based on the mechanisms they use to cope with increased salt uptake: euhalophytes, recretohalophytes, and pseudo-halophytes (Yuan et al., 2019). Euhalophytes actively store toxic ions in their vacuoles, recretohalophytes excrete salt through salt-secretory structures (salt glands), and pseudohalophytes exclude salt when they absorb water through their roots. Euhalophytes also belong to succulent halophytes as they can dilute salt in their succulent leaves or stems (Yuan et al., 2019; Krishnamurthy et al., 2014; Shabala et al. 2012; Song et al., 2008).
There are about 6000 species of terrestrial and marine plants classified as halophytes, of which about 1100 are distributed in the Mediterranean (Lombardi et al., 2022; Glenn et al., 1999; Lielh et al., 1993). The Croatian flora, which belongs to Mediterranean region, contains several halophytic species, such as sea fennel (Crithmum maritimum L.), sea beet (Beta vulgaris L. ssp. maritima (L.) Arcang.), golden samphire (Limbarda crithmoides (L.) Dumort.), marsh samphire (Salicornia europaea L.), sea purslane (Halimione portulacoides L. Aellen), etc. (Dítě et al., 2018; Cutler and Russel, 2004).
Sea fennel (Crithmum maritimum L.), also known as rock samphire or samphire, is an edible perennial plant, the only species of the genus Crithmum belonging to the Apiaceae (Umbelliferae) family. This facultative hydro- and succulent halophyte is usually found in large groups on sea rocks, piers and sandy beaches, along the Mediterranean, Black Sea and European Atlantic coasts (Generalić Mekinić et al., 2016; Meot-Duros and Magné, 2009; Grlić, 2005; Bocchieri and Marchioni-Ortu, 1983). Sea fennel is used in salads, sauces, soups, pickled, and consumed as a condiment, while the infusions prepared from the aerial parts of the plant are used in traditional medicine for prevention, treatment and relief of various diseases such as gastrointestinal disorders, coughs, colds and urogenital disorders (Pedreiro et al., 2023; Renna et al., 2018). Although the whole plant is edible, the succulent leaves, which are rich in carotenoids, polyphenols, vitamin C, ω-3 and ω-6 fatty acids, iodine, organic acids and other bioactive compounds, are the most consumed parts of the plant (Bianco et al., 2018; Grigoriadou et al., 2008). The main group of phenolic compounds found in sea fennel are hydroxycinnamic acids; the plant contains particularly high concentrations of chlorogenic acid and its derivatives (Politeo et al., 2023a; Politeo et al., 2023b; Generalić Mekinić et al., 2018; Siracusa et al., 2011). Due to its richness in bioactive compounds, sea fennel is considered to have various biological activities, such as antioxidant, anti-inflammatory, antimicrobial and enzyme inhibitory activities (Pedreiro et al., 2023; Politeo et al., 2023a; Politeo et al., 2023b; Renna et al., 2018; Generalić Mekinić et al., 2016; Siracusa et al., 2011).
This work aimed to investigate the differences in the chemical composition and biological properties of sea fennel collected from three different locations: a river, an estuary and the sea, in order to show the influence of the harvesting location on the richness of phenolic compounds and antioxidant activity.
2. Materials and methods
General
All reagents were of analytical grade. Prior to extraction, the plant materials were freeze-dried using the FD-1D-50 freeze dryer (Shanghai Bilon Instrument Co. Ltd, Shanghai, PRC). Microwave-assisted extraction was performed using a microwave extraction system (Ethos X, Milestone Srl, Italy). All spectrophotometric assays were tested for phenolic content, including antioxidant assays (DPPH•, FRAP and NO•) using a UV/VIS spectrophotometer (Specord 200 Plus, Analityk Jena GmbH, Jena, Germany). Liquid chromatography analyses were performed with a high-performance liquid chromatograph equipped with a diode array detector (Ultimate 3000, Thermo Fisher Scientific, Wathman, MA, USA) using a Syncronis™ C18 column (250×4.6 mm, 5 μm, Thermo Fisher Scientific, Wathman, MA, USA).
Plant material
Sea fennel samples were harvested in May 2021 from three locations in Omiš (43.4434° N, 16.6929° E, Central Dalmatia, Croatia): near the Cetina shore, near the Cetina estuary and on the Adriatic coast (Figure 1). Pre-treatment prior to freeze-drying and extraction included cleaning and removal of dry leaves and woody parts of the plant and removal of dirt from the plant surface with tap water.

Figure 1. a) Sea fennel harvest locations; b) Sea fennel samples from river shore (A), river estuary (B), and sea shore (C)
Microwave-assisted extraction
The homogenised, dried plant samples were transferred to a round bottom flask filled with distilled water (the ratio of dry matter to water was 1:10). The extraction was carried out at 500 W (94 °C) for
30 minutes (Veršić Bratičević et al., 2023). The prepared extracts were filtered through syringe filters and stored at +4 °C until further analyses.
Spectrophotometric determination of total phenolics, flavonoids and tannins
Total phenolics were determined using the Folin-Ciocalteau method (Amerine and Ough, 1980). For the measurements, which were performed in quintuplicate, 25 µL of the extract was mixed with 1.975 mL distilled water and 125 µL Folin-Ciocalteau reagent in a cuvette. After 5 minutes, 375 µL of 20% sodium carbonate solution was added to the solution, which was then left in the dark for 2 hours. The spectrophotometric measurements were carried out at 765 nm. Total phenolic content (TPC) in the samples was calculated using a chlorogenic acid standard calibration curve and expressed as milligrams of chlorogenic acid equivalents per gram of dry plant material (mg CAE/g DM).
Total flavonoids were determined using the colorimetric method (Shraim et al., 2021). Reaction mixtures of the samples were prepared by mixing 250 µL of extracts, 1.525 mL of distilled water and 75 µL of 5% sodium nitrite solution in a 4 mL cuvette. After 6 minutes, 150 µL of a 10% aluminium chloride solution was added to the mixture. The mixture was allowed to stand for a further 5 minutes, and then 500 µL sodium hydroxide (1 M) was added. The measurements were carried out immediately afterwards at
510 nm.
Total flavonoid content (TFC) was calculated using the quercetin calibration curve and expressed as milligrams of quercetin equivalents per gram of dry plant material (mg QE/g DM).
HPLC-DAD analysis
The chlorogenic acid in sea fennel extracts was identified and quantified by high-performance liquid chromatography using a diode array detector (HPLC-DAD). A gradient consisting of solvent A (2% formic acid), solvent B (acetonitrile) and solvent C (methanol) was applied at a flow rate of 0.8 mL/min as follows: 0 min 96% A, 2% B and C; 40 min 50% A, 25% B and C; 45 min 40% A, 30% B and C; 60 min 50% B and 50% C; 68 min 50% B and 50% C; 70 min 96% A, 2% B and C; 80 min 96% A, 2% B and C. The column was kept at 25 °C and the injection volume was 10 µL.
The chlorogenic acid was identified by comparing the retention time and UV/Vis spectra of the sample peak with the standard and quantified using an external standard calibration curve. The concentration of chlorogenic acid was expressed as milligrams per gram of dry matter (mg/g DM). All samples were analysed in duplicate.
Antioxidant assays
The DPPH• assay is based on the reduction of the 2,2-diphenyl-1-picrylhydrazyl radical by the antioxidants present in the system (Katalinić et al., 2010). For the measurement, 2 mL of DPPH•
(A = 1,2 ± 0,5) reagent solution was added to a cuvette and the absorbance was measured at 517 nm (AC(0)). Then 50 µL of the diluted extract (1:10) was added to the solution. After 1 hour, the absorbance of the reaction solution was measured again (AA(T)). The percentage of inhibition was calculated as follows:
,
where AC(0) represents DPPH• solution absorbance (t = 0 h), and AA(T) represents absorbance of reaction solution (t = 1 h).
FRAP assay is based on the reduction of the colourless [Fe3+-(2,4,6-tris(2-pyridyl)-s-triazine)2]3+ to the bright blue coloured [Fe2+-(TPTZ)2]2+ complex (Benzie and Strain, 1999). 3 mL of FRAP reagent was added to a cuvette and the absorbance was measured at 593 nm. Then 100 µL of extracts were added to the reagent. The absorbance of the reaction solution was measured after 4 minutes and the resulting absorbance was calculated by subtracting the absorbance of the reaction solution from the FRAP reagent absorbance. The results for each sample were calculated using the Fe2+ calibration curve and expressed in mM Fe2+ equivalents.
The NO• assay is based on the spontaneous formation of nitrogen oxide from nitroprusside at physiological pH (Dastmalchi et al., 2008). 0.5 mL of extract and 0.5 mL of sodium nitroprusside solution (10 mM) were mixed in a test tube, incubated at 37 °C for 150 minutes, and stored in a dark place. After 20 minutes, 1 mL of Griess reagent was added to the reaction solution. The absorbance of the reaction solution was measured after 40 minutes at 546 nm.
The percentage of inhibition is calculated as follows:
,
where ASP represents the absorbance of the blank solution, and AE represents the absorbance of the sample.
3. Results and discussion
As mentioned earlier, sea fennel is a facultative halophyte, meaning that it grows well in soils with high salt concentrations, but can also grow well in soils that contain little or no salt (Aslam et al., 2011, Sabovljević and Sabovljević, 2007). This work aimed to determine how growth conditions, especially salt concentration, affect the phenolic content and antioxidant properties of sea fennel. To this end, aqueous extracts obtained by microwave-assisted extraction were tested and plant material was collected from the river shore (A), estuary (B) and sea shore (C).
Total phenolic and flavonoid content
Table 1. Total phenolics (TPC) and flavonoids (TFC) in sea fennel samples harvested at different locations
| Sea fennel sample | TPC (mg GAE/g DM) | TFC (mg QE/g DM) |
|---|---|---|
| River shore | 30.51 ± 0.26 | 2.26 ± 0.02 |
| River estuary | 29.91 ± 0.64 | 1.71 ± 0.02 |
| Sea shore | 25.51 ± 0.16 | 1.68 ± 0.01 |
The content of total phenolics and flavonoids shown in Table 1 indicates the highest concentration of the two phenol groups in the samples harvested at the river shore. The phenolic content of the sample from the river shore was close behind that of the extracts from sea fennel harvested near the river estuary, while no significant difference was found in the total flavonoid concentrations of the extracts from plants growing at the river estuary and at the sea.
Plant phenolics, one of the most important groups of secondary metabolites, have a number of different functions in plant organisms (Zhang et al., 2022). However, one of their most important functions is plant defense: they are produced in large quantities when plants are exposed to biotic and abiotic stresses. The synthesis and accumulation of phenolics is influenced by various external and internal factors such as trauma, drought, nutrient stress, pathogen attack and others (Bhattacharya et al., 2010). Pungin et al. (2022) conducted a study on two obligate halophytes (Spergularia marina L. Griseb. and Glaux maritima L.) to investigate the relationship between soil salinity and plant phenolic content and found that the content of total phenolics initially increased with the water salinity, reaching the highest value at a salinity of 75 mM, which could be equated with brackish water.
However, with increasing salinity, the phenolic content continued to decrease and reached the lowest value at the highest salt concentration. In contrast to these results, in our study, the highest concentration of total phenolics was found in extracts of sea fennel from the river bank with the lowest salinity, while the lowest concentrations of total phenolics were present in the plants exposed to the highest salinity stress. Ksouri et al. (2007) investigated the effects of salinity stress on two varieties of the halophyte Cakila maritima. The results showed that the phenolic content of C. maritima var. Jerba initially increased with increasing salinity and doubled when salinity increased from 0 to 100 mM. However, a further increase in salinity led to a decrease in total phenolics in the plant. The total phenolics in C. maritima var. Tabarka was highest in the plant not exposed to salinity stress and also decreased with increasing salinity. Reginato et al. (2014) studied the effect of salinity stress on Prosopis strombulifera and found that the flavonoid and phenolic content of this Argentine halophytic shrub increased when the plant was exposed to higher salinity.
Determination of chlorogenic acid
As for secondary metabolites, sea fennel is known for its richness in hydroxycinnamic acids, especially chlorogenic acid (Politeo et al., 2023a; Politeo et al., 2023b, Generalić Mekinić et al., 2018; Siracusa et al., 2011). For this reason, in this study, special emphasis was also placed on the chromatographic determination of chlorogenic acid.
The highest concentration of chlorogenic acid, as shown in Figure 2, was found in the extract from the plants harvested near the river estuary (1.64 mg/g), followed by the extract from the plant harvested on the river bank (1.34 mg/g), while the lowest concentration was found in the extract from the plant growing near the sea shore. Pungin et al. (2022) also reported that the hydroxycinnamic acids content increased with soil salinity, but then decreased when the plant was exposed to higher salinity stress.
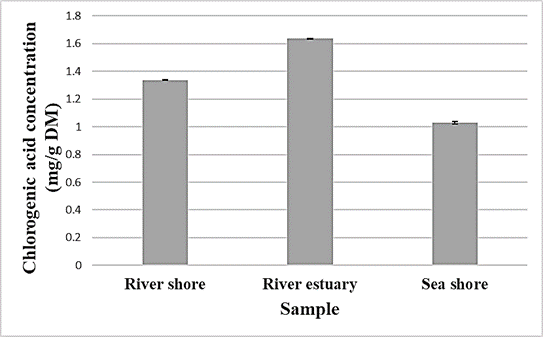
Figure 2. Chlorogenic acid concentrations in sea fennel samples harvested at different locations
Antioxidant activity
In order to draw conclusions about the antioxidant properties of investigated sea fennel extracts, they were tested using three antioxidant methods based on different mechanisms; Ferric Reducing Antioxidant Power (FRAP), 2,2-diphenyl-1-picrilhydrazil (DPPH•) scavenging activity, and Nitric Oxide (NO•) scavenging activity (Table 2).
Table 2. Antioxidant activity of the sea fennel samples
As can be seen, the extract from the estuary showed the highest antioxidant activity detected by the DPPH and FRAP methods, followed by the extracts from the river and sea shore samples.
As shown in Table 2, the results for inhibition of NO radical, expressed as a percentage, differed from the results of the other two tests for antioxidant activity.
Interestingly, the extract of the plant growing at the sea shore showed the highest inhibition of NO• (48.7%), while it had the lowest FRAP and DPPH activities.
The sample harvested at the river estuary had the highest FRAP and DPPH activities but also the highest concentration of chlorogenic acid. Chlorogenic acid and its derivatives, the predominant phenolic compounds in sea fennel, are known for their antioxidant activity in vivo and in vitro. In addition, studies have confirmed that they have the potential to alleviate oxidative stress in various disease models (Liang and Kitts, 2015). Since the results show a correlation between the tested antioxidant activities and the concentration of chlorogenic acid, it can be deduced that chlorogenic acid is the main compound responsible for the antioxidant activity of sea fennel samples.
5. Conclusion
The results show that the harvest location of sea fennel has a significant influence on its phenolic composition and antioxidant activity. Sea fennel grown at the location with the lowest salt concentration (the river shore) had the highest concentration of total phenolics and flavonoids, but not the highest antioxidant activity, while the sample from the river estuary had the highest concentration of chlorogenic acid and the highest FRAP value and DPPH• inhibition, which may indicate that chlorogenic acid is the main compound responsible for the antioxidant activity of the plant. The richness of phenolic compounds and the high biological activity of sea fennel prove that it is a suitable subject for further research and use in the cosmetic, medicinal and pharmaceutical industries.
Author Contributions: Conceptualization, N.M. and I.G.M.; methodology, N.M., D.S. and I.G.M.; formal analysis, N.M., D.S. and P.B.; investigation, N.M. and I.G.M.; data curation, N.M. and P.B.; I.G.M.; writing-original draft preparation, P.B.; writing-review and editing, N.M., I.G.M., D.S. and P.B.; visualization, P.B.; supervision, I.G.M.
Funding: This work is part of the PRIMA programme supported by the European Union. Project title: “Innovative sustainable organic sea fennel ( Crithmum maritimum L.)-based cropping systems to boost agrobiodiversity, profitability, circularity, and resilience to climate changes in Mediterranean small farms” (acronym: SEAFENNEL4MED) (https://seafennel4med.com/).
Acknowledgments: The authors are also thankful for the scientific-research equipment financed by the EU grant “Functional integration of the University of Split, PMFST, PFST and KTFST through the development of the scientific and research infrastructure” (KK.01.1.1.02.0018).
Conflicts of Interest: The authors declare no conflict of interest.
