1. Introduction
Mycotoxins are low-molecular metabolites formed by certain strains of mould species mainly from the genera Aspergillus, Penicillium, and Fusarium. They can enter the food chain by direct or indirect contamination of food. The real danger of mycotoxins is reflected in their longterm action, because they can cause various mutagenic and cancerous changes at the cellular level (Knasmüller et al., 2001; Clark et al., 2006; Venkatesh and Keller, 2019). At the same time, it is impossible to avoid their presence in food, and thus, the exposure to mycotoxins.
Plant polyphenols are relatively well known for their antioxidant, anti-mutagenic, anticancer, anti-inflammatory, antiangiogenic, antiulcer and antimicrobial properties. Based on preliminary studies of the antigenotoxicity of extracts of various plant species, it was observed that those species that are rich in polyphenols have a significant antigenotoxic effect based on the prevention of oxidative damage caused by cytostatics in the culture of human lymphocytes (Kaefer et al., 2008; Mikulášová et al., 2005). In addition, as one of the most economical raw materials rich in polyphenols, so far primarily used for nutrition and obtaining tannic extracts, chestnut (Castanea sativa Mill.), whose extracts have been poorly tested, can be explored for potential antioxidative and antigenotoxic potentials. The addition of such extracts of potential antigenotoxic effect to food could reduce the toxic effects due to the exposure to mycotoxins.
Many substances of plant origin have been shown to "capture" free radicals, which are known to cause oxidative stress. Chestnut leaf - Castanea sativa Mill. is a good example of such plant species that contains a number of bioactive substances with potential antioxidant properties. Species of the Castanea Mill. genus are known to be immunomodulators, whose phytochemical composition indicates the presence of substances with antioxidant and antigenotoxic effects (Živković et. al., 2008; 2010; Mujić et al., 2011).
Polyphenols from the group of phenol-carboxylic acid derivatives are associated with the prevention of diseases caused by oxidative stress, such as malignant, cardiovascular and neurodegenerative diseases (Mikulášová et al., 2005). It is assumed that polyphenols with their antioxidant action reduce the formation of free radicals that significantly contribute to the cytotoxic and genotoxic effect of certain mycotoxins (Costa et al., 2007; Kopjar et al., 2007; Jukić et. al., 2021).
The aim of this study was to investigate whether extracts of Castanea sativa Mill. leaf extracts are antigenotoxic in vitro. The results determined the concrete effect of the extract, as well as the most dominant components of C. sativa after treatment of cells with mycotoxins aflatoxin B1 and ochratoxin A.
2. Materials and methods
Plant material and chemicals
Plant material
Air-dried sweet chestnut leaves were used as material. The samples were collected in one locality from a large number of trees in the vicinity of Bihać, Una-Sana County – Bosnia and Herzegovina. Air-dried and pulverized plant material (20.00 g) was extracted with 200 mL of 70% ethanol using an ultrasonic bath for 30 minutes.
The extract was filtered and the residue was then re-extracted with 200 mL of the same solvent as described above. Obtained extracts were combined and then concentrated to dryness under vacuum at 50 °C using a rotary evaporator.
Chemicals
Acetic acid, aluminium chloride, formic acid, disodium hydrogen phosphate, ethanol, ethylenediaminetetraacetic acid (EDTA), hexamethylenetetramine, methanol, pyrogallol, sodium carbonate, sodium citrate, sodium dihydrogen phosphate, sodium hydroxide, sodium nitrite, sodium phosphate, sulphuric acid, tannic acid (95%), thiourea were purchased from Kemika (Zagreb, Croatia). Acetonitrile (HPLC grade) was purchased from J.T. Baker (Deventer, Netherlands). Ammonium molybdate, gallic acid, protocatechinic acid, p-coumaric acid, ellagic acid, quercetin, kaempferol kemferol, apigenin, rutin, catechin, epikatehin, casein, 2-deoxy-D-ribose, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulfonic acid sodium salt (ferrozine), 2,2-diphenyl-1-picryl-hydrazyl (DPPH•), hydrogen peroxide, potassium ferricyanide, rosmarinic acid (96%), sodium acetate, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), dimethylsulfoxide (DMSO) and sodium molybdate were obtained from Sigma-Aldrich (St. Louis, MO, USA). Butylated hydroxytoluene (BHT, ≥ 99%), iron (II) chloride and quercetin-3-rutinoside (rutin, ≥ 95%) were obtained from Fluka (Buchs, Switzerland). Ascorbic acid (99%) and trichloroacetic acid (TCA) were purchased from Acros Organics (Geel, Belgium), Folin–Ciocalteu’s phenol reagent, 3-tert-butyl-4-hydroxyanisole (BHA) and 2-thiobarbituric acid (TBA) were obtained from Merck (Darmstadt, Germany). Iron(III) chloride and hydrochloric acid were obtained from Riedel-de Haën (Seelze, Germany) and POCh (Gliwice, Poland), respectively.
Qualitative and quantitative analysis of C. sativa leaf extract
Determination of total polyphenols, tannins and flavonoids, phenolic acid
Determination of total tannin as well as total polyphenol contents was performed following the method described in European Pharmacopoeia (EDQM, 2004). Briefly, the extract (0.5 g) was boiled for 30 min in a water bath (150 mL), then the filtrate was made up to 250 mL with distilled water and the obtained solution served as stock solution. An aliquot of stock solution was mixed with Folin–Ciocalteu’s phenol reagent and sodium carbonate solution. After 30 min, the absorbance was read at 720 nm (A1), and the quantification of total phenols was done with respect to the standard calibration curve of pyrogallol (6.25-50.00 µg). For the determination of tannins content, stock solution was vigorously shaken with hide powder for 60 min. Since the hide powder adsorbed tannins, phenols unadsorbed on hide powder were measured in filtrate, after addition of Folin–Ciocalteu’s phenol reagent in a sodium carbonate medium (A2). The percentage content of tannins, expressed as pyrogallol, was calculated from the following equation:
where A3 is the absorbance of the test solution containing 0.05 g of pyrogallol, and m the mass of the extract (g).
The total flavonoid contents of tested plant extract were determined using the spectrophotometric method of Christ and Müller (1960). Each powdered plant sample (0.2 g) was mixed with 20 mL of acetone, 2 mL of 25% hydrochloric acid and 1 mL of 0.5% hexamethylenetetramine solution and heated under reflux in a water bath for 30 min. The extract was filtered and re-extracted twice with 20 mL of acetone for 10 min. Filtrates were combined and made up to 100 mL with acetone. An aliquot of 20 mL of the acetone extract was mixed with 20 mL of water and then extracted with three quantities, each of 15 mL, of ethyl acetate. Combined ethyl acetate layers were washed twice with water then filtered and diluted to 50 mL. To 10 mL of this solution 0.5 mL of 0.5% solution of sodium citrate and 2 mL of 2% aluminium chloride solution (in 5% methanolic solution of acetic acid) was added and then diluted to
25 mL with 5% methanolic solution of acetic acid. The mixture was allowed to stand for 45 min and the absorbance was measured at 425 nm.
A sample solution prepared in the same manner, but without addition of aluminium chloride solution, served as a blank. All determinations were performed in triplicate.
The percentage content of flavonoids, expressed as quercetin, was calculated from the equation:
where A is the absorbance of the test solution at 425 nm and b is the mass of the sample, in grams.
Determination of total phenolic acids
Determination of hydroxycinnamic acid derivatives was performed according to procedure described in European Pharmacopoeia (EDQM, 2004). Briefly, 0.2 g of the powdered plant material was extracted with 80 mL of 50% ethanol under a reflux condenser in a boiling water bath for 30 min. The cooled extract was filtered, the filter rinsed with ethanol, and then combined filtrate and rinsing was diluted to 100 mL with 50% ethanol. An aliquot of 1 mL of the extract was mixed with 2 mL of 0.5 M hydrochloric acid, 2 mL of Arnow reagent (10% aqueous solution of sodium nitrite and sodium molybdate), and 2 mL of 8.5% sodium hydroxide and diluted to 10 mL with water. The absorbance of the test solution was measured immediately at 505 nm against sample blank. The percent of total hydroxycinnamic acid content was calculated and expressed as rosmarinic acid, according to the following expression:
where A is the absorbance of the test solution at 525 nm and m is the mass of the sample, in grams. Analysis of each sample was performed in triplicate.
High Performance Liquid Chromatography Analysis (HPLC) of C. sativa leaf extract
Herbal extracts were analyzed according to the method described by Belščak-Cvitanović et al. (2011). The samples were filtered through a 0.45 μm filter (Nylon Membranes, Supelco, Bellefonte, USA) before High Performance Liquid Chromatography (HPLC) analysis. 20 μL of each sample was injected for HPLC analysis using a Varian Pro Star Solvent Delivery System 230 (Varian, Walnut Creek, USA) and a Photodiode Array detector Varian Pro Star 330 (Varian, Walnut Creek, USA) by using a reversed-phase column Gemini-NX C-18 column (Phenomenex, USA) (150×4.6 mm, 2.6 μm i.d.). The solvents consisted of 3% formic acid in acetonitrile (solvent A) and 3% formic acid in water (solvent B) at a flow rate of 0.9 mL/min. The elution was performed with a gradient starting at 10% A, rising to 40% A after 25 min, then to 70% A after 30 min and becoming isocratic for 5 min. Chromatograms were recorded at 278 nm. Detection was performed with a Photodiode Array Detector by scanning between 200 and 400 nm, with a resolution of 1.2 nm. Phenolic compounds were identified by comparing the retention times and spectral data with those of standards. The data acquisition and treatment were conducted using Star Chromatography Workstation Version 5 software. All analyses were repeated three times.
Antioxidant activity of C. sativa leaf extract
2, 2-Diphenyl-1-picrylhydrazyl radical (DPPH•) radical scavenging assay
The free radical scavenging activities of the samples were measured using the stable DPPH• radical, according to the method of Blois (1958). Briefly, 0.1 mM solution of DPPH in ethanol was prepared and 1 mL of this solution was added to 3 mL of sample solution in ethanol at different concentrations (0.39-200 µg/mL). The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was then measured at 517 nm.
The capability to scavenge the DPPH• radical was calculated using the following equation:
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of sample, corrected for the absorbance of sample itself. Butylated hydroxytoluene (BHT) was used for comparison. All determinations were done in triplicate.
Hydroxyl radical (OH•) scavenging assay
Hydroxyl radicals were generated by a Fenton reaction (Fe3+-ascorbate-EDTA-H2O2 system), and the scavenging capacity towards the hydroxyl radicals was measured by using a deoxyribose method as described by Halliwell et al. (1987) with a slight modification. The reaction mixture contained, in a final volume of 1 mL, 2-deoxy-2-ribose (2.8 mM), phosphate buffer (0.1 mM, pH 7.4), iron (III) chloride
(20 μM), EDTA (100 μM), hydrogen peroxide (500 μM), ascorbic acid (100 μM) and various concentrations (12.5-1600 μg/mL) of the test sample or reference compound. After incubation for 1 h at 37 °C, an aliquot of the reaction mixture (0.5 mL) was added to 1 mL of 2.8% TCA solution, followed by 1 mL of TBA solution (1% in 50 mM sodium hydroxide) and then the mixture was heated 20 min at
90 °C to develop the colour. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. All experiments were performed in triplicate. Hydroxyl radical scavenging activity was evaluated with the inhibition percentage of 2-deoxyribose oxidation by hydroxyl radicals, according to the following equation:
where: A0 is the absorbance of the control without a sample, A1 is the absorbance in the presence of the sample and deoxyribose and A2 is the absorbance of the sample without deoxyribose. Thiourea was used as a positive control.
Electron spin resonance (ESR) measurements
Hydroxyl radical scavenging activity
As hydroxyl free radicals (•OH) are highly reactive, with relatively short half-lives, the concentrations found in natural systems are usually inadequate for direct detection by electron spin resonance (ESR) spectroscopy. Spin-trapping is a chemical reaction that provides an approach to help overcome this problem. Hydroxyl radicals are identified due to their ability to form nitroxide adducts (stable free radicals form) from the commonly used DMPO as the spin trap (Buettner, 1985). The Fenton reaction was conducted by mixing 200 μL of DMPO (112 mM), 200 μL of DMF, 200 μL of H2O2 (2 mM) and 200 μL of FeCl2 (0.3 mM) (control). The influence of C. sativa extract on the formation and stabilization of hydroxyl radicals was investigated by adding investigated extracts in the Fenton reaction system at the range of concentrations 10-1000 μg/mL. ESR spectra were recorded after 5 minutes, with the following spectrometer settings: field modulation 100 kHz, modulation amplitude 0.226 G, receiver gain 5 x 105, time constant 80.72 ms, conversion time 327.68 ms, center field 3440.00 G, sweep width 100.00 G,
x-band frequency 9.64 GHz, power 20 mW, temperature 23 °C.
The SAOH value of the extract was defined as:
where h0 and hx are the height of the second peak in the ESR spectrum of DMPO-OOH spin adduct of the control and the probe, respectively.
Superoxide anion radical scavenging activity
Superoxide anion radicals (O2•-) were generated in the reaction system obtained by mixing 500 µL of dry dimethylsulfoxide (DMSO), 5 µL of KO2/crown ether (10 mM / 20 mM) prepared in dry DMSO and 5 µL of 2 M DMSO solution of DMPO as spin trap. The influence of extracts on the formation and transformation of superoxide anion radicals was obtained by adding the DMF solution of C. sativa extract to the superoxide anion reaction system at the range of concentrations 5-100 g/mL. After that the mixture was stirred for 2 min and transferred to a quartz flat cell ER-160FT. The ESR spectra were recorded on an EMX spectrometer from Bruker (Rheinstetten, Germany) under the following conditions: field modulation 100 kHz, modulation amplitude 4.00 G, receiver gain 1×104, time constant 327.68 ms, conversion time 40.96 ms, center field 3440.00 G, sweep width 100.00 G, x-band frequency 9.64 GHz, power 20 mW, temperature 23 °C.
The SAo2●- value of the extract was defined as:
where h0 and hx are the height of the second peak in the ESR spectrum of DMPO-OOH spin adduct of the control and the probe, respectively.
Comet assay
This test was performed using whole human blood in the volume of 5 mL that was taken by venipuncture from a forty-year-old male volunteer (non-smoker). Blood was mixed with cell culture media RPMI 1640 (1:1) and transferred into 96-well plates. Prior to cell treatment water extract of C. sativa was sterilised by filtration trough millipore filter (0.2 nm). Aflatoxin B1 (AFB1) was dissolved in dimethyl sulfoxide (DMSO), while absolute ethanol was used as solvent for OTA. Leukocytes were exposed for 2 hours to plant extracts at concentrations 1 mg/mL (K1), 10 mg/mL (K2) and 20 mg/mL (K3), single AFB1 (3 µM) and OTA (10 µM), as well as combination of each mycotoxin with each concentration of plant extract. Water (10%), DMSO (0.03%) and ethanol (0.3%) were used as controls.
The comet assay was carried out according to Singh et al. (1988). After cell treatment, aliquots of 20 μL of cell suspension were mixed with 80 μL 0.5% low melting point agarose (LMP), and 100 μL of agarose-cell suspension was spread onto a fully frosted slide (Surgipath, Richmond, Il, USA) pre-coated with 1.5% normal melting point agarose, NMP (in Ca- and Mg-free PBS buffer). The slides were allowed to solidify on ice for 10 minutes. After overnight lysis at 4 °C in a mixture of 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris (pH 10) supplemented with 1% Triton-X, the slides were placed in denaturation and electrophoresis buffer (10 mM NaOH, 200 mM Na2EDTA, pH 13), incubated for 20 min, and electrophoresed for 20 min at 25 V and 300 mA. DNA was neutralised with a neutralisation solution (0.4 M Tris/HCl, pH 7.5) three times 5 min each. The slides were kept in a humid atmosphere in a dark box at 4 °C until further analysis. For image analysis, DNA was stained with 100-250 μL ethidium bromide solution per slide for 10 min. Slides were scored using an image analysis system (Comet assay II, Perceptive instruments Ltd., U.K.) connected to a fluorescence microscope (Zeiss, Germany). Images of 100 randomly selected cells were measured. Only comets with a defined head were scored. Comet parameters considered in this study were the tail length, the proportion of DNA in the comet tail (tail DNA or tail intensity), and tail moment, which was calculated as the product of the fraction of DNA in the comet tail and the tail length.
3. Results and discussion
The total amount of polyphenols in Castanea sativa
Castanea species were investigated regarding their polyphenolic content and separation. The presence of flavonoids, phenolic acids and tannins in ethanolic plant extracts was detected by spectrophotometrical method. The results of spectrophotometric determination of the total amount of polyphenols (prepared as shown in chapter 2.2) are presented in the Table 1.
The determination of the amount of polyphenols in the species C. sativa was performed with the spectrophotometrical method. Table 1 shows the values of the content of the specimens of the ethanol extract in C. sativa and the estimated content of the total polyphenols, tannin, phenolic acid, flavonoids.
Table 1. Contents of phenolic acids, flavonoids, tannins and total polyphenols in
C. sativa selected Castanea species
| Contens (%) | ||||
|---|---|---|---|---|
| Plant extracts | Total polyphenols | Flavonoids | Phenolics acids | Tanins |
| Castanea sativa | 6.74±0.09 | 0.43±0.025 | 0.44±0.12 | 2.09±0.25 |
Each value is the mean ± SD of three indenpendent measurements
Phytochemical analysis of polyphenolic compounds
A large number of HPLC methods for determining phenolic compounds in plant extracts have been published. Essentially, they are adapted to determine the content of the most dominant polyphenols in one plant species or a certain number of compounds of that class in a variety of extracts (Harnly et al., 2006; Jukić et al., 2021). Given the large number of compounds belonging to the flavonoid group, as well as the fact that most are related in the form of glycosides, it is difficult to find the ideal method for determining their total content. Flavons and flavonoids (quercetin, luteoline, apigenin, routine) in such biological substrates most often appear in glycosylated form, so a specific glycoside is also needed to identify them. There are a large number of works relating to the examination of the composition of polyphenol C. sativa (Živković et al., 2009; Mujić et al., 2011).
Chromatogram of lyophilized chestnut extract and some identified compounds are shown in Figure 1. Similar to the results of other authors (Basile et al., 2000; Almeida et al., 2008; Živković et al., 2009; Živković et al., 2010), chestnut leaf is rich in gallic and ellagic acid derivatives, and especially ellagitannin. Ellagitannins were not identified in this paper due to the lack of commercial standards. The order of elution of the components under the described chromatographic conditions is: gallic acid derivatives, protocatechinic acid, p-coumaric acid, rutin, ellagic acid, quercetin derivatives, kaempferol derivatives, and apigenin. Protocatechinic acid and rutin are present only in traces, so it was not possible to carry out their quantification. Chromatographic analysis determined the presence of ellagic acid and its derivatives which indicate the presence of tannin components in chestnut leaf samples.
The values of the share of ellagic acid derivatives in the chestnut leaf extract (Table 2), as well as gallic acid derivatives, kaempferol and quercetin are in the range of the results of most previous research (Basile et al., 2000; Živković et al., 2009; Živković et al., 2010).
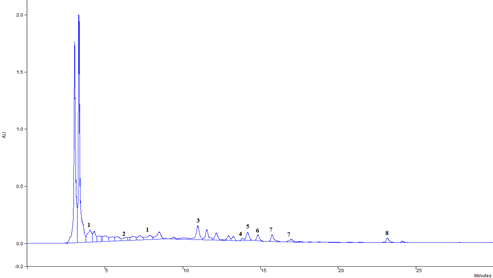
Figure 1. Chromatogram of extract Castanea sativa leaf: 1- gallic acid derivatives, 2- protocatechinic acid, 3- p- coumaric acid, 4- rutin, 5- ellagic acid, 6- quercetin derivatives, 7- kaempferol derivatives, 8- apigenin
The different values of the content of polyphenolic components in chestnuts can be explained by the action of a number of factors, including hereditary properties, the difference in the maturity of the plant and numerous environmental factors (Dorman et al., 2003).
Table 2. Polyphenol components in C. sativa leaf extract
Considering the listed identified compounds that are most abundant in the chestnut leaf, this can certainly be reflected in the desired expected properties of preventing the genotoxic effect of the mycotoxins tested in this paper. Intensive research is focused on testing the anti-carcinogenic properties of ellagic acid. The ability of ellagic acid to inhibit metabolic activation of carcinogens and protect DNA from alkylation was discovered (Das et al., 1985; Barch et al., 1988). Agullo et al. (1994) reported that quercetin has a cytotoxic effect against colon cancer cells and leukemia cells in vitro. During a 25-year study, which included seven countries, an inverse correlation was found between the intake of flavonols and flavones and a decrease in mortality from cardiovascular diseases (Hertog et al., 1995). Animal studies confirm that the intake of quercetin in food reduces the incidence of breast and lung cancer (Verma et al., 1988; Khanduja et al., 1999).
Antioxidant activities of Castanea ethanolic extracts
The antioxidant activities of polyphenols were attributed to their redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers, as well as their metal chelating abilities. Polyphenolic compounds such as flavonoids, phenolic acids and tannins are considered to be the major contributors to the antioxidant activity of medicinal plants, fruits and vegetables (Rice-Evans et al., 1996; Pereira et al., 2009). The ability to remove free radicals mostly depends on the structural properties of phenol compounds such as the energy of dissociation of O-H bound, the delocalisation of phenol radicals (PheO*) and steric disturbances caused by the substitutes on the aromatic ring (Sanchez-Moreno et al., 1998). Therefore, in the present study four different assays were employed in order to determine and compare the antioxidant properties of selected Castanea species, as well as to elucidate their mode of action.
Unlike free radicals generated in a lab, such as superoxide and hydroxyl radicals, the use of free stable radicals is an advantage, since it is not influenced by the secondary reactions, such as chelate with metals and enzyme inhibition, caused by additives (Yamaguchi et al., 1998).
After measuring absorptions at 517, the percentage of the inhibition capacity of DPPH• radicals were calculated. According to the research results, the effectiveness of DPPH radical scavenging with chestnut leaf extract increases rapidly with increased concentration and reaches almost 90% radical inhibition at 25 μg/mL. Concentrations higher than 1.56 μg/mL are needed to achieve 50% inhibition, and already at concentrations above 25 μg/mL the effect of the extract approaches the effect of quercetin and gallic acid, which are characterized by strong antiradical activity. The ethanolic extract of C. sativa species has a strong ability to neutralize DPPH radicals judging by the low IC50 value (Table 3). The effect of the extract in this concentration is about 1.3 times stronger compared to the reference antioxidant, and yet three times weaker compared to the standards of quercetin and gallic acid. According to the DPPH radical scavenging ability expressed by the IC50 value, the following order of the tested samples was determined: quercetin > gallic acid > catechin > chestnut leaf extract > BHA. It should be noted that the inhibition of DPPH radicals by catechin stagnated from a concentration of 3.13 μg/mL and upwards. The obtained results show that the extract of chestnut leaves has a strong ability to capture free radicals and encourage the continuation of more detailed research on antioxidant activity and the isolation and characterization of bioactive polyphenolic components.
The results are in accordance with the values determined by Mujić et al. (2011), where a concentration of sweet chestnut leaf extract of 50 μg/mL caused 89% inhibition of DPPH radicals. However, they found a significantly higher IC50 of 9.2 μg/mL (Table 3). Barreira et al. (2008) have determined an IC50 value for DPPH of 170 μg/mL. Quercetin standard had the highest DPPH radical inhibition activity, and its great activity as a ‘’radical scavenger’’ was recognized before (Farombi et al., 2005; Marzouk et al., 2006). Gallic acid reached 90% neutralization of DPPH at 25 μg/mL, while Gašo-Sokač et al. (2011) determined 61 μg/mL for gallic acid using the same method. For gallic acid as a reference standard, Orhan and Üstün (2011) have determined 91% inhibition only at a concentration of 500 μg/mL or 92% at 1000 μg/mL. The antioxidant activity of the extract can also be attributed to other polyphenolic components such as flavonoids, phenolic acids or phenolic diterpenes (Pietta et al., 1998; Velioglu et al., 1998; Burda and Oleszek, 2001; Cervellati et al., 2002; Živković et al., 2010).
The main components of the sweet chestnut leaf extract (Table 2) lead to the conclusion that in the case of neutralization of the stable DPPH radical, quercetin and gallic acid were largely responsible for the antiradical activity of the extract, given their abundance.
The DPPH assay has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. Nitrogen centered radicals such as DPPH• react with phenols via two different mechanisms: direct abstraction of phenol H-atoms and electron transfer processes. The contribution of one or the other pathway depends on the nature of solvent and/or the redox potentials of the species involved. DPPH• is a stable free radical compound with a characteristic absorption at a wavelength of 517 nm. Antioxidants upon interaction with DPPH• either transfer an electron or hydrogen atom to DPPH•, thus neutralizing its free radical character. The colour of the reaction mixture changes from purple to yellow with a decrease of the 517 nm absorbance. The degree of discolouration indicates the scavenging potential of the antioxidants (Foti et al., 2004; Villaño et al., 2007).
The research of Yokozawa et al. (1998) has shown that tannins and some flavonoids show an activity in relation to DPPH• radicals and that the activity is closely related to their chemical structure. With the increase in galiol groups, the molecular mass and ortho-hydroxy groups in the structure, the activity of tanine increases, and the number and position of hydroxyl groups represent an important characteristic of flavonoids for ''quenchers'' free radicals.
Hu et al. (2004) released the results of the study of the 'scavangers' activity on DPPH radicals of water-methanol extracts of more then 300 medicinal herbs. For 56 of the examined specimens they got IC50 values under 0.500 mg of the specimen/mL of the extragent. The same authors attribute the activity of DPPH• radicals of plants to the present flavonoids and tannins in the extract.
When examining the content of antiradical activity while considering the antioxidant potential of the sample, it is also necessary to take into account the fact that the extracts are a complex mixture of natural compounds and that their antiradical activity is not the result of the activity of only one component. Very often, the compounds interact with each other and the overall profile of phytochemicals could determine the functionality as a result of the synergistic action of the individual constituents (Vattem et al., 2005).
Table 3. Comparative overview of IC50 values as well as total antioxidant capacities of Castanea ethanolic extracts, polyphenolic compounds and reference antioxidants
| IC50* (µg/mL) | ||
|---|---|---|
| DPPH scavenging activity | OH scavenging activity | |
| C. sativa extract | 1.88 | 92.9 |
| Quercetin | 0.58 | - |
| Catechin | 1.29 | 289.6 |
| Gallic acid | 0.62 | 1596.9 |
| BHA | 2.69 | - |
| Thiourea | - | 40.9 |
* IC50 value: concentration at which the DPPH and OH radicals were scavenged by 50%. Each value is expressed as mean ± SD. (n = 3); − not tested.
The method based on the determination of the catching ability of OH• free radical was used, according to the principle of oxidising deoxyribose when exposed to the hydroxyl radicals which appear with the Fenton's reaction. In the reaction, the hydroxyl radicals appear with the decomposure of H2O2, whereby the high potential of EDTA-Fe2+ causes the decomposure of deoxyribose. The oxydative decomposure can be perceived by heating up products with 2-thiobarbituric acid (TBA) in acidic conditions, whereby the pink chromogene (TBARS, thiobarbituric acid reactive species), which has the absorption maximum at 532 nm. The added antioxidants compete with the deoxyribose for the hydroxyl radicals and reduce the amount of chromogenes (Cheng et al., 2003).
Sweet chestnut leaf extract had a higher ability to scavenge the free radical OH• compared to pure polyphenolic components, and almost identical activity to standard antioxidants. Catechin showed a weaker antiradical activity than thiourea and the extract, but was again noticeably more effective than gallic acid. At the highest tested concentration of catechin, 20% weaker OH• inhibition was found compared to thiourea and extract. Negative values for gallic acid indicate stimulation of the formation of this radical. Gallic acid derivatives are among the most abundant components of chestnut leaf extract (Table 2), and the result is interesting in the context of the effectiveness and application of the extract, although the implications are not entirely clear.
Sweet chestnut leaf extract neutralizes the DPPH radical better than the hydroxyl radical (Table 3), and part of the observed effect may be attributed to the pro-oxidant action of gallic acid derivatives.
Given the above, and according to the determined IC50 value (Table 3), thiourea was again confirmed as the best scavenger of OH•, while the C. sativa leaf extract had about two times less antiradical ability, followed by catechin with seven times, and gallic acid even 40 times less antiradical ability.
Findings from Electronic Spin Resonance
One part of our investigation on antioxidant activity of C. sativa extract was the scavenging activities on hydroxyl and superoxid anion radicals measured by ESR method. Using a spin trap, such as DMPO, it is possible to convert reactive hydroxyl radicals to stable nitroxide radicals (DMPO -OH adducts) with spectral hyperfine splitting that reflects the nature and structure of these radicals. The reaction of Fe2+ with H2O2 in the presence of the spin trapping agent DMPO generated a 1:2:2:1 quartet of lines with hyperfine coupling parameters ( aN= aH= 14.9 G) (Čanadanović-Brunet, et al., 2005). The intensity of the ESR signal, corresponding to the concentration of free radicals formed, was decreased in the presence of different amounts of C. sativa extract. The total elimination of hydroxyl radical (SAOH=100%) was obtained in the presence of 1000 μg/mL of extract, which indicates that this applied concentration inhibits the creation of hydroxyl radicals completely. This was confirmed by the calculated IC50 values of 43 μg/mL.
Comparing the intensity of the ESR signal of the DMPO-OOH spin adduct blank test and the sample, it was determined that aqueous solutions of Castanea extract lyophilisates in the range of tested concentrations have an inhibitory effect on O2• - formation or participate in their transformation. The IC50 value of C. sativa extract (8.5 μg/mL) shows that extract is rich in antioxidant compounds and efficiently scavenge superoxide anion radicals.
According to research by Almeida et al. (2008), mean IC50 values of chestnut leaf extract for scavenging O2•- radicals were 13.6 µg/mL.
Moreover, and according to other authors, the properties of the ESR signals obtained in the hypoxanthine/xanthine oxidase (X/XO) generator system of this radical with the use of DEPMPO (5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide) show that all of the examined extracts (leaf, catkin, spiny bark, chestnut bark, brown skin, chestnut seed and fruit) have exhibited O2•- radical inhibition activity (Živković et al., 2010). The values of the relative inhibition of O2•- radicals were: 73% for sweet chestnut, 80% for the chestnut leaf of Lovran chestnut, and 86% for the leaf of grafted Italian chestnut in a concentration of 200 µg/mL. Živković et al. (2008) also investigated the capacity of sweet chestnut extract to eliminate lipid peroxidation. It was determined that all extracts protected liposomes from lipid peroxidation. In addition, the extracts can be used as protective factors by inhibiting the oxidative stress caused by ˙OH and H2O2, and at the same time they are extremely resistant to the effects of the radicals of this degradation process. For these reasons, the effect of lipid peroxidation on their cleaning ability is minimal.
Inhibition of ROS and RNS by chestnut extracts was evaluated in vitro and confirmed by other authors in the context of testing the possible significance in application as antioxidant formulations (Almeida et al., 2008). It has already been documented that the chestnut leaf (Calliste et al., 2005) as well as the chestnut seed (Vázquez et al., 2008) have phenolic compounds with significant antioxidant capacity.
Comet assay
Table 4 shows the results of alkaline comet assay following 2 hours exposure to various concentrations of C. sativa extracts (K1, K2, K3), single AFB1 and OTA as well as combination of extracts and mycotoxins. Plant extracts did not exert genotoxic activity taking into account values of tail intensity and tail moment. However, significant increase in tail length was observed upon exposure to the highest concentration of plant extract ( P<0.05), while lower concentrations did not provoked the increase of this parameter.
Table 4. Evaluation of primary DNA damage measured in human leukocytes following 2-h exposure to C. sativa extracts and single AFB1 and OTA or combinations of each mycotoxin with plant extracts
C1, C2, C3 – control solvent; SD- standard deviation; 25%- P- 25% percentile; M – median; 75% -P- 75% percentile; *- compared to control ( P<0.05); **- compared to AFB1 or OTA given alone ( P<0.05)
Since both AFB1 and OTA are food contaminants with genotoxic activity the purpose of this study was to see whether their genotoxic action could be antagonised if leukocytes are simultaneously exposed to this mycotoxins and water extracts of C. sativa.
Chestnut leaf extract in the lowest concentration is not genotoxic, judging by the results for tail length (Table 4). However, higher concentrations significantly reduce median tail lengths compared to control. Similarly, other authors have confirmed that the comet’s tail moment (product of tail length and % DNA in the tail) was positively correlated with the extent of DNA breakage, whereas it was negatively correlated with the level of DNA crosslinks. Thus, an increase in the tail moment can be interpreted as an increase or a decrease in DNA breaks in the cell, if crosslinks are formed between the chains or if DNA breaks are reduced (Collins et al., 1995; Ashby et al., 1995). The same authors have observed the influence of quercetin on the DNA damage of human lymphocytes caused by MNNG and observed an increase in the tail moment at lower concentrations of quercetin and its decrease at higher concentrations. Quercetin derivatives were identified in the tested sweet chestnut extract (Table 2). In addition to the already mentioned direct binding to DNA (Ferguson, 2001; Gibellini et al., 2010), quercetin can promote oxidative DNA damage, especially in the presence of transition metal ions (Kuo et al., 1998).
As it was expected, exposure to AFB1 at 3 µM and OTA at 10 µM significantly increased all three comet parameters comparing to control solvents ( P<0.05) (Table 4).
According to the results for chestnut leaf extract shown in Table 4, leukocytes that were simultaneously exposed to AFB1 and the two highest concentrations of the extract, have significantly lower median tail lengths. At the same time, the tail intensity was significantly lower except in the case when AFB1 was added simultaneously with the lowest concentration of the extract. The lower tail moment compared to cells exposed to mycotoxin alone also shows the neutralizing effects of the plant extracts.
Sweet chestnut leaf extract in the three tested concentrations also significantly reduced tail length compared to cells exposed to OTA (Table 4). At tail intensity, the lower two concentrations suppressed the genotoxic effect, in contrast to the highest concentration. In the case of the tail moment, a significant decrease was recorded only at a maximum of two concentrations (Table 4).
Natural components in food, including various polyphenols, show potent antioxidant activity, and therefore could prevent OTA toxicity (Baldi et al., 2004; Renzulli et al., 2004). In addition, research into the antioxidant and antigenotoxic effects of three different chestnut extracts (catkin, leaf, spiny burs) was conducted on Rin-5F β-cells of rat pancreas (Mujić et al., 2011). Cells were treated with streptozotocin alone (STZ, a substance that induces β-cell death and causes diabetes), or together with sweet chestnut extracts. The analysis of the degree of DNA damage using the comet assay revealed a large extent of damage in the sample treated with STZ, which was reflected in the tail moment. Rin-5F cells treated with a combination of STZ and extracts have shown a lower degree of DNA damage. It was also concluded that chestnut leaf extract provided the best protection of cellular DNA from oxidative and genotoxic effects.
After many laboratory and epidemiological studies, it has been found that diet is responsible for approximately 35% of all human cases of cancer (Doll and Peto, 1981). Based on epidemiological research, Block et al. (1992) and Steinmetz et al. (1996) have estimated that the incidence of cancer can be reduced by at least 20-30% with a healthy diet. The central role of diet in preventing carcinogens has also been confirmed by the World Cancer Research Foundation (World Cancer Research Fund, 2007). The interactions between diet and the biological processes that lead to cancer are very complex. Although a large number of carcinogenic substances have been found in food, the human body has its own defense mechanisms that are sufficient if the exposure is not quantitatively excessive and chronic. Plants rich in flavonoids and phenolic components are known for their anticancer, antioxidant and other biological activities. Polyphenols can reduce or inhibit the mutagenic potential of mutagens and carcinogens (Miadokova et al., 2008). Controlling cell mutation with natural antimutagens can result in a variety of ways to prevent mutations that are essential, both in the case of cancer and in diseases caused by genotoxic agents (Birt et al., 2001).
Author Contributions: H. J. formal analysis, writing-original manuscript draft preparation, review editing, and ESR analysis. D. K. performed the HPLC and voltammetric measurements, data collection, formal analysis. M. Š. K.: writing - review and editing. N. K.: Comet assay. K. D.: writing - original draft preparation. I. K.: project conceptualization, supervision, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding: Ministry of Education, Science, Culture, and Sports of the Una-Sana Canton - Bosnia and Herzegovina.
Acknowledgments: The authors acknowledge the financial support of Ministry of Education, Science, Culture, and Sports of the Una-Sana Canton, Co-financing of scientific research and research and development projects of special interest to the Una-Sana Canton (03-02-2190-647/2023).
Conflicts of Interest: “The authors declare no conflict of interest.”
4. Conclusion
The strong antioxidant activity of the Castanea sativa extract was determined, as well as the dominant components (ellagic acid), which indicate the ingredients that mostly contribute to the effect of the extract. Suppression of the genotoxic effect of AFB1 and OTA by the comet assay was also determined, where a significant reduction in tail length, tail intensity and tail moment was found in leukocytes simultaneously treated with mycotoxins and different concentrations of extracts. The addition of the tested extracts with a potential antigenotoxic effect to food could therefore, in addition to its role in increasing the sustainability of the food itself through antioxidant action, modulate the toxicity of the mycotoxins present after consumption.
5. Literature
flavonoids and phenolic acids. Free Radical Biology and Medicine 20, 933-956. https://doi.org/10.1016/0891-5849(95)02227-9
| © 2019 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0). |
