Microphytobenthos
The microphytobenthos (MPB) colonises the surface sediment layer (2 to 3 mm thick) where strong chemical-physical gradients exist (MacIntyre et al. 1996). In temperate regions, the microphytobenthic community is mainly composed of benthic diatoms, which are either colonial or unicellular, either free-living or attached to a substrate through gelatinous protrusions and play an important role in primary production in marine ecosystems (Falkowski et al. 2004). Classification based on sediment typology reveals distinct groups: (i) epipelon, diatoms living on sediment, (ii) epipsammon, diatoms adhering to sand grains, and (iii) epiphyton, diatoms living on other photosynthetic organisms (Round et al. 1990). The microphytobenthic community plays a key ecological role within the aquatic ecosystem, regulating nutrient and oxygen fluxes at the water-sediment interface and contributing significantly to primary production (MacIntyre et al. 1996). Due to their ecophysiological characteristics, these microorganisms serve as valuable indicators for water quality assessment and in paleoecological reconstructions (Stevenson and Pan 1999, Cibic and Blasutto 2011, Barinova et al. 2019, B-Béres et al. 2023).
Microphytobenthic populations, particularly in shallow aquatic environments, are frequently enriched by phytoplanktonic species that settle on sediments in conditions devoid of turbulent movement, tidal currents, or water column stratification. Conversely, under conditions of pronounced turbulence in the water column, microphytobenthic organisms may be resuspended, infiltrating the phytoplankton community (Delgado et al. 1991, MacIntyre et al. 1996). Furthermore, identical species may be present both in the water column and in the surface sediments, making it difficult to characterize the typical species in each habitat ecologically (Cibic et al. 2022). In a two-year study (2003-2004) on MPB biodiversity conducted in Northern Adriatic Sea, on average, 9% of the species in the sediment were planktonic (Cibic et al. 2007a); however, they were predominantly in poor condition. Only species with robust frustules, such as Pseudo-nitzschia seriata, were identifiable and likely still photosynthetically active (Cibic et al. 2007b).
In recent years, the application of light microscopy (LM) and scanning electron microscopy (SEM) has enabled the discovery of numerous diatom species in marine coastal waters, greatly improving our understanding of diatom communities in different coastal regions, such as the Black Sea, the Mediterranean Sea or Yellow Sea (Park et al. 2018, Kaleli and Akçaalan 2021, Zidarova et al. 2022). While various authors have previously compiled checklists (Hendey 1974, Viličić et al. 2002, Cibic and Facca 2010, Caraus 2017), the recent and progressing systematics of diatoms has resulted in many synonyms and the transfer of taxa to a new species of a genus (Li et al. 2018, Morales et al. 2019). A considerable proportion of the studies conducted has predominantly focused on planktonic forms of diatoms, thus the publications considered herein generally include both benthic and planktonic diatoms.
Mediterranean Sea
The Mediterranean Sea is a semi-enclosed continental sea that is almost entirely landlocked between Europe, Africa and Asia and consists of several sub-zones, including the Alboran Sea, the Balearic Sea, the Ligurian Sea, the Tyrrhenian Sea, the Ionian Sea, the Adriatic Sea, the Aegean Sea and the Levantine Basin. It is a concentration basin that receives relatively low saline Atlantic Water (AW), which flows eastwards in the surface layer and gains salt due to the positive Evaporation-Precipitation balance over the Mediterranean (Menna et al. 2022). The northeastern Mediterranean Sea is influenced by the brackish waters of the Black Sea through the Sea of Marmara. The shallowest part of the Mediterranean Sea is the northernmost third of the Adriatic Sea (i.e., the Northern Adriatic Sea), a shelf with depths up to 100 m (Vilibić et al. 2023). The Mediterranean Sea is considered an oligotrophic basin with a few biological production and biodiversity hotspots (Myers et al. 2000). The meadows of Posidonia oceanica L. Delile, a species of seagrass endemic to the Mediterranean Sea, are the most important ecosystem in the Mediterranean in terms of biodiversity. However, many alien and invasive species have recently been reported along the eastern coasts of the Mediterranean and Aegean Sea, originating from the Red Sea through the Suez Canal (Zenetos et al. 2005). The Mediterranean Sea is an important basin, and its coasts have been heavily influenced by humans through tourism, urbanization and maritime transport. Additionally, the Mediterranean Sea is considered a hot spot of global warming, as it has been changing faster than the global ocean, with recent studies agreeing on increases in sea level, sea surface temperature and salinity over the past two decades, which have had a strong impact on the marine environment (Menna et al. 2022).
As far as modern diatom studies in the Mediterranean Sea are concerned, one of the pioneering studies was published by Witkowski et al. (2000). While Witkowski et al. (2000) published a monograph of diatom taxa from various marine coastal sites worldwide, Blanco and Blanco’s monograph (2014) showed widespread taxa alongside site-specific taxa in the Mediterranean region. In addition, several checklists with a combination of planktonic species in the Mediterranean Sea have been published. Viličić et al. (2002) published a phytoplankton checklist for the eastern Adriatic Sea, while Kaleli and Akçaalan (2021) showed the diatom flora of the Turkish coasts, Tas (2013) investigated the phytoplankton composition in the Aegean Sea (Tas 2013), and Velâsquez and Cruzado (1995) investigated the diatom flora of the northwestern Mediterranean Sea. Most of the studies used a similar sampling technique, specifically the use of a plankton net designed for phytoplankton sampling, with incidental observations of benthic diatoms. Consequently, the use of the plankton net resulted in the observation of relatively large cell sizes for the most commonly cited taxa.
In recent decades, the literature on the distribution of MPB species in the Mediterranean Sea has been limited to the coastal regions of Spain and France (Delgado 1989, Barranguet et al., 1996, Barranguet 1997, Riaux-Gobin et al. 1998). While these publications highlight the importance of MPB as a primary producer within coastal or lagoon ecosystems, and report the total MPB expressed as chlorophyll and pigment concentrations, they do not provide information on the taxonomic composition or present a comprehensive taxonomic list. In recent study, Pérez-Burillo et al. (2022) evaluated the advantages and limitations of LM and DNA metabarcoding for the identification of benthic diatom communities in shallow coastal environments of the Mediterranean Sea using biofilm samples from different substrates in the Ebro delta bays (Spain). The compilation of the historic records of marine and brackish diatom species and genera reported for the French coastal areas since 1888 to 2019, with information on the geographical locations and ecology (e.g. sampled substrate, habitat, growth forms) for most taxa by Ribeiro et al. (2022), provides a great dataset for the benthic marine diatoms of French Mediterranean coast.
Before the compilation of the MPB checklist for the Italian seas (northern and western Middle Adriatic, and Ligurian Sea) by Cibic and Facca (2010), there were no time series data (no continuous observations at specific sites over an extended period with constant abundance) on the MPB in the Italian seas. The knowledge of MPB in Italian seas is low compared to longer-term studies on phytoplankton (Cibic and Facca 2010). The list of Cibic and Facca (2010) encompasses taxa derived from natural sediment samples collected from various locations, including the Gulf of Trieste (Sdrigotti et al. 1999, Welker et al. 2002, Cibic et al. 2007a), the Venice Lagoon (Tolomio et al. 1999, 2002, Facca et al. 2002a, b, 2003, 2004, Tolomio 2004, Facca and Sfriso 2007), the Adriatic coast from Ancona to the Po Delta (Totti 2003), the Lesina Lagoon (Gambi et al. 2003) as well as epibiontic microalgae on marine hydroids Eudendrium racemosum in the Ligurian Sea (Romagnoli et al. 2007, 2014), epilithic diatoms on different artificial hard substrates in the Conero area (Totti et al. 2007), and the first colonisation stages of diatoms on artificial hard substrates at two stations in the Gulf of Trieste (Bartole et al. 1991-94) and the Venice Lagoon (Tolomio and Andreoli 1989, Tolomio et al. 1991). Additionally, several recent papers provided benthic diatom checklists either in the main text or as Supplementary Material. These studies cover the Gulf of Trieste (Franzo et al. 2014, Rogelja et al. 2018) and the Grado Lagoon (Natali et al. 2023), the Adriatic coast from Ancona to the Po Delta (Accoroni et al. 2016, Cibic et al. 2019), and the Venice Lagoon (Baldassarre et al. 2023). Additionally, microbenthic community structure and trophic status of sediments of the Ionian Sea were studied in the period 2013–2014 (Rubino et al. 2016). The only available information regarding the microphytobenthic community of the Ligurian Sea refers exclusively to epibiontic species associated with marine hydroids (Romagnoli et al. 2007, 2014), showing that diatoms living on this marine invertebrate derive an advantage from both the host and the environmental conditions due to the availability of nutrients and organic compounds (Cibic and Facca 2010). Diatom assemblages from the Tyrrhenian Sea have been used to study the effects of heavy metal contamination, where this group showed the presence of teratogenic forms in response to this kind of pollution (Rogelja et al. 2016).
The epiphytic diatom communities of the endemic Mediterranean seagrass P. oceanica are among the most thoroughly studied (Mazzella et al. 1994, De Stefano et al. 2000). Mazzella et al. (1994) identified Cocconeis Ehrenberg species as the most abundant and frequent diatoms on the leaves in all seasons and throughout the depth range of the seagrass distribution. De Stefano et al. (2000) analysed eight species of Cocconeis on the leaves of P. oceanica using SEM on samples collected around the island of Ischia (Gulf of Naples, Italy). Their results showed that certain Cocconeis species such as C. scutellum var. posidoniae M.De Stefano, D.Marino & L.Mazzella and C. neothumensis var. marina M.De Stefano, D.Marino & L.Mazzella exhibit high abundance, forming a continuous, almost monospecific layer on the colonised segments of the leaves (Mazzella et al. 1994). In a comparative study, De Stefano et al. (2008) provided additional insights into C. scutellum Ehrenberg and its varieties and presented information on the geographical distribution of all the analysed C. scutellum taxa, including from the leaves of Posidonia in the Mediterranean region collected in spring 2005 and 2006 (the season when the density of epiphytic macroalgae is low) and covering almost the entire Mediterranean Sea (including sampling sites along the coasts of Spain, Italy, Slovenia, Croatia, Greece and Türkiye).
A comprehensive review of the existing literature reveals that there is only a limited number of studies focused on the coastal waters of Türkiye (Kaleli and Akçaalan 2021). The Sea of Marmara and the Aegean Sea were the most intensively studied areas, with 20 and 19 studies, respectively. Studies on benthic and planktonic species composition and biogeographic distribution in the Mediterranean Sea and the Black Sea were rather sparse with only 11 and 14 studies, respectively (Kaleli and Akçaalan 2021). On the Turkish coast of the Aegean Sea, Altuğ et al. (2011) and Aslan et al. (2018) investigated the composition of phytoplankton in the northeastern Aegean Sea. Koray (2001) published a checklist of phytoplankton in the Turkish seas. These studies focused mainly on the planktonic forms of diatoms. Several studies have also been carried out on the benthic diatoms of the shores of Greece (Economou-Amilli 1980, Belegratis 2002, Belegratis and Economou-Amilli 2002, Louvrou 2007). Foged (1985a, b) published monographs on the diatom communities of the islands of Samos, Kos and Kalymnos. In the southern Aegean Sea, periphyton colonisation in hydrothermal marine areas of the island of Milos has been studied (Louvrou 2007). The author later described Detonia dobrinae Louvrou, Danielidis & Economou-Amilli (Louvrou et al. 2006) and several taxa of Meloneis I.Louvrou, D.B.Danielidis & A.Economou-Amilli (Louvrou et al. 2012). Loir (2010-2014) published micrographs of diatoms from the Mediterranean Sea, including on the island of Crete. Tas (2013) observed that diatoms and dinoflagellates were the dominant groups of phytoplankton groups on the Datça peninsula. Cocconeis and Amphicocconeis M.De Stefano & D.Marino have been studied extensively in the Aegean Sea (De Stefano and Marino 2003, De Stefano et al. 2006, 2008, Majewska et al. 2014). These studies introduced several new species and expanded knowledge of these genera using both LM and SEM. Recently, Konucu and Develi (2021) reported two new monoraphid diatom species, Cocconeis sigillata Riaux-Gobin et Al-Handal and Amphicocconeis rodriguensis Riaux-Gobin et Al-Handal, from samples collected in the northeastern Mediterranean Sea.
Morphological studies by Pennesi et al. (2011, 2012) investigated the ultrastructure of some marine Mastogloia Thwaites ex W.Smith species collected during opportunistic sampling of benthic diatoms from diverse geographical regions, including tropical, subtropical including Indonesia and the Red Sea, and temperate zones, such as Patmos Island in the Aegean Sea, Greece, where epiphytic diatom samples were collected from seagrasses and macroalgae in September 2000. The genus Mastogloia has been reported in Turkish temperate inland waters, exhibiting a presence in both pelagic and benthic diatom flora (Elmacı and Obalı 1998, Akbulut and Yıldız 2002, Çelekli and Külköylüoğlu 2006, Sıvacı et al. 2007, Çolak Sabancı 2013). Recently, several papers have been published on diatom communities in the Aegean Sea; Li et al. (2018) described Gedaniella flavovirens (H.Takano) Chunlian Li, A.Witkowski & M.P. Ashworth in the Fragilariaceae, and several taxa were introduced from the Aegean Turkish coasts in the Iztuzu coast of Muğla (Kaleli et al. 2020). Additionally, diatoms from Iztuzu beach and Iztuzu coastal lake (Kaleli 2019, Kaleli 2022), as well as the benthic diatom composition in Homa Lagoon (Izmir) (Çolak Sabancı 2010, 2011, 2012a, b, 2013, Çolak Sabancı and Koray 2010), have been studied. Some of these studies introduced new records for the Turkish flora, while others documented diverse compositions.
In the African Mediterranean region, on the other hand, there have been very few studies. Aleem (1950) contributed new taxa from the Levantine Basin of Egypt. Voigt (1963) worked on Mastogloia species in the Mediterranean. Zalat (2001, 2002) and Zalat et al. (2019) investigated the community structure of the Suez Canal and Egyptian coasts, describing new species and showing their spatial distribution, focussing on habitat preferences and life forms. Their studies revealed that most species are cosmopolitan, with a significant presence of freshwater forms. More recently, a study on the distribution of diatoms on the Algerian coasts has been published (Kaddeche et al. 2022).
Hydrographic and oceanographic characteristics of the Adriatic Sea
The Adriatic Sea, the northernmost embayment of the Mediterranean Sea, 800 km long and 150 km wide, divided into three sections (Northern, Middle and Southern), is under considerable continental influence and characterised by diverse water masses (Viličić et al. 2002, Vilibić et al. 2023, Verri et al. 2024). Water exchange with the Mediterranean Sea takes place through the 800 m deep Strait of Otranto, with significant inflows into the Southern Adriatic Sea in winter, driven by the "Bimodal Oscillating System" (BiOS). This internal mechanism influences the biogeochemistry and biology of both the Adriatic and Ionian Seas (Civitarese et al. 2010, 2023, Ljubimir et al. 2017). The BiOS connects the deep thermohaline cell of the eastern Mediterranean, which originates in the Southern Adriatic, with the Northern Ionian Gyre (NIG) and causes decadal shifts in circulation and redistribution of salinity (Civitarese et al. 2023). The eastern Adriatic coast is influenced by high-salinity and nutrient-poor inflows from the Ionian Sea and freshwater discharges from oligotrophic karstic rivers (Batistić et al. 2014, Ljubimir et al. 2017). Saline waters enter the Adriatic Sea in intermediate layers on the eastern side of the strait, while the Adriatic water mainly exits via the western shelf and the deep section of the strait (Gačić et al. 1996, Vilibić et al. 2023). The nutrient levels in the Ionian Sea and the Adriatic Sea show similar variability, although the Adriatic Sea generally has a lower nutrient content. By contrast, anticyclonic NIG enhances the inflow of fresher, nutrient-rich Modified Atlantic Water (MAW) originating in the Western Mediterranean, causing nutricline upwelling at the NIG border, decreasing Southern Adriatic temperature, salinity, and density, and reducing winter convection, thereby increasing ecosystem productivity (Civitarese et al. 2010). An overview of productivity across the geographical regions of the Adriatic Sea shows that the Northern Adriatic, particularly the Gulf of Trieste, Po River delta, and Rovinj area, has been the focus of most studies on primary production (N=46). The Middle Adriatic has been notable for in situ primary production measurements since the 1960s. Annual primary production ranges from 87.4–260.0 g C m⁻² y⁻¹ in the Northern Adriatic and 70.0–177.4 g C m⁻² y⁻¹ in the Middle Adriatic. The Southern Adriatic is the least studied, with only daily estimates available (236–374 mg C m⁻² d⁻¹) (Matek and Ljubešić 2024). The occurrence of organisms from the Atlantic/Western Mediterranean and the Eastern Mediterranean/temperate zones in the Adriatic correlates with the decadal circulation changes (the anticyclonic and cyclonic circulations) of the NIG (Civitarese et al. 2010, 2023).
Review of diatom investigations in the Adriatic Sea
The study of diatoms in the Adriatic Sea began in the late 1800s and several species were included in the diatom flora like Bacillaria adriatica Lobarzewski (Lobarzewski 1840). One of the pioneering studies in the Adriatic region was conducted by Kützing, who described Berkeleya adriatica Kützing (Kützing 1844) and Amphitetras adriatica Kützing (Kützing 1845). This was followed by the work of Grunow (1860), Cleve and Grunow (1880), and Van Heurck (1881), who discovered additional new species. In the 1900s, Mastogloia adriatica Voigt was described by Voigt (1963), and these records were followed by local studies in the 2000s.
In recent decades, several authors have published studies on phytoplankton composition, such as Viličić et al. (2002) with a checklist of phytoplankton in the eastern Adriatic Sea, Viličić (2014) with a book on the ecology and composition of phytoplankton in the Adriatic, Neri et al. (2024) with a comparative analysis of phytoplankton diversity in the Northern Adriatic Sea using microscopy and metabarcoding etc. According to Viličić et al. (2002), marine diatoms, comprising 518 species (330 pennates and 174 centric), were the predominant phytoplankton group in the eastern Adriatic. The study highlighted a regional distribution, with pennate diatoms more abundant in the Northern Adriatic and centric diatoms dominating the offshore Southern Adriatic.
In the Adriatic Sea, benthic diatoms have been reported from several areas, including: the Gulf of Trieste (Bartole et al. 1991-94, Sdrigotti et al. 1999, Munda 2005, Cibic et al. 2007a, b, Cibic and Blasutto 2011, Franzo et al. 2014, Rogelja et al. 2018), Venice Lagoon (Tolomio and Andreoli 1989, Tolomio et al. 1999, Facca et al. 2002a, b, 2003, 2004, Tolomio et al. 2002, Facca and Sfriso 2007, Baldassarre et al. 2023), the northwestern Adriatic coast (Totti 2003, Totti et al. 2007, Franzo et al. 2015, Accoroni et al. 2016, Pennesi and Danovaro 2017, Cibic et al. 2019, Natali et al. 2023) and the eastern Adriatic coast (Burić et al. 2004, Miho and Witkowski 2005, Caput Mihalić et al. 2008, Levkov et al. 2010, Car et al. 2012, 2019a, b, 2020, 2021, Mejdandžić et al. 2015, Nenadović et al. 2015, Hafner et al. 2018a, b, Kanjer et al. 2019, Seveno et al. 2023, 2024).
The Northern Adriatic Sea, the most intensively studied area (e.g. Sdrigotti et al. 1999, Totti 2003, Munda 2005, Totti et al. 2007, Facca and Sfriso 2007, Mejdandžić et al. 2015), is a shallow sub-basin of the Adriatic Sea characterized by a morphologically complex coastline leading to the formation of variable hydrodynamic and sedimentary environments. The hydrology in this region is influenced by various factors such as winds and river discharge, and the general circulation is cyclonic. Freshwater (mean flow of 1496 m3 s-1 in the period 1917-2008, Cozzi and Giani 2011) originates from major rivers along the northern and northwestern coasts, with the Po River contributing nutrient-rich water, constituting approximately one-third of the total freshwater input into the Adriatic Sea. The Po's freshwater flow, characterized by relatively fresh and mesotrophic water, might form a thin surface layer over the northern sub-basin in summer, while it is reduced in winter and flows directly south along the Italian coast (Poulain 2001 and references therein). An analysis of the most recent decade (2013–2022) shows a pronounced negative trend across the entire Adriatic Sea, indicating a consistent annual reduction of −4.2% in freshwater input throughout the river basin (Aragão et al. 2024). The sedimentation pattern is consistent with the hydrodynamic circulation (Ravaioli et al. 2003) and shows a narrow strip of recent sand along the coast, followed by a broad belt of muddy sediments. Offshore, there is a muddy transition zone characterized by a gradual increase in sand content, dominating a wide-open sandy shelf area with minimal recent sedimentation (known as relict sands) (Franzo et al. 2015 and references therein). Although the Northern Adriatic shelf is a relatively low-energy environment with low tidal ranges and wave heights, ephemeral deposition of sediments occurs after high-tide events and is subsequently remobilized by waves in dense currents (Traykovski et al. 2007). Sediments migrate southward in a series of wind-induced resuspension events promoted by the Bora and Scirocco winds (Fain et al. 2007). The main sources of biogenic elements are both autochthonous (plankton) and allochthonous (atmospheric inputs and soil organic matter transported by rivers) factors (Franzo et al. 2015). In line with the findings of Totti (2003), Franzo et al. (2015) also reported the presence of the centric diatom Paralia sulcata (Ehrenberg) Cleve in sediments off the coast of Emilia-Romagna (Northern Adriatic) in a study aimed at improving the understanding of offshore benthic communities in the Adriatic Sea. Cibic et al. (2012) studied benthic diatom community dynamics in relation to temperature, salinity, nutrient concentrations, freshwater inflow, and mucilage in the Gulf of Trieste (Northern Adriatic Sea, Italy) over seven years (1999–2005) at two sublittoral stations. They found that Nitzschia and Navicula showed a positive trend with increasing temperature, while Pleurosigma exhibited a negative trend. Navicula and Nitzschia appeared to be negatively affected by mucilage events in the summers of 2000 and 2004, while Diploneis occupied the ecological niche temporarily vacated by Navicula and Nitzschia. Diploneis had a negative relationship with temperature at the shallower site. Although no significant correlations between bin-averaged salinity and diatom abundance were found, Cylindrotheca was observed at high salinity levels. Despite the general seasonal variability observed in benthic diatom communities, characterized by distinct summer and winter assemblages (Cibic et al. 2012), Franzo et al. (2015) did not observe significant temporal variability in microalgal composition.
In the Venice Lagoon, benthic diatoms from the surface sediment layer were studied to investigate possible relationships between epiphytic diatoms and water quality in shallow coastal areas characterised by marked physical and chemical gradients and significant anthropogenic impacts (Facca and Sfriso 2007). In addition, Cibic and Blasutto (2011) investigated the response of diatoms to different nutrient concentrations under nutrient-rich conditions in a benthic diatom study at three sublittoral sites in the Gulf of Trieste (Italy). It can be observed that the dominance of a single species leads to a decline in diversity. Under oligotrophic conditions, without providing a competitive advantage for a single species when diatoms faced limitations by more than one nutrient, diversity remained high (Cibic and Blasutto, 2011). This study suggests that benthic diatoms, like macrobenthos, can serve as useful indicators of nutrient enrichment and represent a potential and innovative tool for biomonitoring.
The most interesting environments along the Middle Adriatic are highly stratified estuaries formed by several small karstic rivers (Zrmanja, Krka), which are maintained by sufficient river discharge and low tides (Viličić et al. 2002). In the oligotrophic and highly stratified Zrmanja estuary, the composition and abundance of diatoms in biofilm formed on artificial substrates exposed at different depths were studied in July 2000 (Burić et al. 2004, Caput Mihalić et al. 2008). The study conducted by Caput Mihalić et al. (2008) focused on the composition and abundance of diatoms within biofilms on artificial substrates exposed at different depths in the Zrmanja estuary in July 2000. The predominant periphyton species identified were Amphora coffeaeformis (C.Agardh) Kützing and Navicula veneta Kützing. Shannon-Wiener index ranged from 0.87 to 2.08 at the upper estuary station and from 1.1 to 2.7 at the other station. Additionally, Burić et al. (2004) investigated the abundance of periphytic pennate diatoms, in particular Cocconeis scutellum, attached to artificial substrates in the karstic Zrmanja estuary in the summer of 2000. The authors focused on comparing the abundance of attached Cocconeis cells with those suspended in the planktonic phase.
Previous research focused mainly on the Northern Adriatic and estuaries (e.g. Burić et al. 2004, Caput Mihalić et al. 2008, Levkov et al. 2010), resulting in a significant knowledge gap regarding the diatom flora of the Southern Adriatic, especially before 2008. Although part of the material for the taxonomic study by Levkov et al. (2010) came from the Ombla River estuary near Dubrovnik (the Southern Adriatic), it focused mainly on 15 species of the genus Rhoicosphenia based on LM and SEM. A literature review revealed not only a lack of studies in the coastal waters of the Southern Adriatic before 2008, but also the absence of continuous long-term observations of benthic diatoms at a consistent location. There was also a shortage of information on the seasonal distribution and succession of epiphytic diatoms on macroalgae. The most comprehensive study of diatom community structure in the Southern Adriatic was conducted between autumn 2008 and autumn 2010 in areas inhabited by invasive Caulerpa species (Car et al. 2012, 2019a, b).
Prior to the studies by Car et al. (2012, 2019a, b), there were no studies on diatom communities associated with the marine green macroalga Caulerpa taxifolia (M.Vahl) C.Agardh (“killer seaweed", Bryopsidales, Chlorophyta), which outcompetes native seaweeds and seagrasses in the Mediterranean Sea by forming dense carpets, leading to biodiversity loss. The study by Kanjer et al. (2019) provided valuable insights into the biodiversity of threatened P. oceanica meadows, affected by human activities and invasive species, while also providing crucial information for the identification of taxa-specific epiphytic diatom communities in the eastern Adriatic seagrass meadows. This study describes the epiphytic diatom community living on the blades of the P. oceanica in the coastal region of the eastern Adriatic Sea (Dugi Otok island). LM analysis of 21 samples revealed a diverse epiphytic diatom community with 68 taxa belonging to 30 genera across all depths (43 from 10 m depth, 41 from 15 m depth and 39 from 20 m depth), dominated by the genus Cocconeis, which is typical of the epiphytic growth on the leaves of P. oceanica. All identified species are common in other marine benthic periphytic and epiphytic habitats. Although there was no positional influence on community structure, ANOSIM tests (P < 0.05) indicated significant depth-related differences.
In contrast to the study of Kanjer et al. (2019), the study by Car et al. (2019a) focused on the taxonomy of epiphytic diatoms associated with invasive macroalgae of the genus Caulerpa. These macroalgae are characterised by secondary metabolites such as caulerpenyne (CYN), which act as chemical defence mechanisms against herbivores and epiphytes (Box et al. 2008, 2010). It is interesting to note that the CYN content varies between the different Caulerpa species, e.g. Caulerpa prolifera has higher CYN concentrations than invasive Caulerpa species under similar climatic conditions (Box et al. 2010). Epiphytic diatoms were studied in the summer and autumn of 2010 on the eastern Adriatic coast (Hvar Island) on fronds of C. taxifolia and, for comparison, on the autochthonous macroalgae Padina sp. and Halimeda tuna (J.Ellis & Solander) J.V.Lamouroux. Qualitative analysis was performed using LM and SEM. The Shannon-Wiener diversity index determined for C. taxifolia showed a wide range of values (3.11-4.88), with a maximum in August and a minimum in October. While the number of taxa on the fronds of C. taxifolia increased from June (41) to August (88), it decreased in autumn due to the high relative abundance of Cocconeis caulerpacola Witkowski, Car and Dobosz, a diatom typical of Caulerpa. On the other hand, the highest number of taxa on Padina sp. was observed in September (82). The detailed composition of the epiphytic diatoms and the seasonal dynamics in the area affected by the invasive macroalga Caulerpa taxifolia were determined for the first time.
In a study by Car et al. (2012), a new diatom species, C. caulerpacola, was observed on the green alga C. taxifolia over a wide geographical range; from the Adriatic (Stari Grad Bay, Hvar Island, Middle Adriatic, Croatia), on the Mediterranean coasts (Saint Raphaël, west of Cannes, France), and on the east coast of Australia (Moreton Bay, southeast Queensland). It has also been observed on Caulerpa cylindracea Sonder (syn. C. racemosa var. cylindracea (Sonder) Verlaque, Huisman & Boudouresque), another Mediterranean invasive alga. LM, SEM and TEM (transmission electron microscopy) were used for morphological and fine structure analyses. The new species was compared with closely related species (C. borbonica, C. diruptoides and C. pseudodiruptoides). Surprisingly, C. caulerpacola was commonly found on C. taxifolia despite its reputation as a 'killer seaweed' in very high abundance, but its occurrence seems to be very patchy. Indeed, C. taxifolia seems to be a suitable host for epiphytic diatoms, especially for the tiny Cocconeis caulerpacola.
Furthermore, a study by Car et al. (2019b) compared the epilithic diatom community structures at three sites on the eastern Adriatic coast (Croatia) in the presence of two different invasive Caulerpa species, C. taxifolia and C. cylindracea, which have spread rapidly in the Mediterranean in recent decades. Between November 2008 and October 2010, 40 samples were collected seasonally at three sites (Hvar Island, Mljet Island and Dubrovnik). Qualitative analyses using LM and EM identified 310 epilithic taxa from 65 genera. The predominant genera were Mastogloia (48), Amphora (32), Diploneis (24), Nitzschia (23), Navicula (22) and Cocconeis (19). A similar seasonal shift in growth forms was observed at all sampling sites, with a doubling (Hvar, Mljet) or tripling (Dubrovnik) of the number of erect diatoms in spring. Apart from erect forms, Dubrovnik and Mljet were characterised by adnate forms, while Hvar was characterised by tube-dwelling forms. The highest values of the Shannon-Wiener diversity index occurred in autumn and ranged from 5.26 to 5.34. Significant differences in diatom communities among sampling sites were correlated with the presence of invasive macroalgae (Hvar - C. taxifolia; Mljet and Dubrovnik - C. cylindracea). The main taxa contributing to the community variance were Cocconeis scutellum var. scutellum, Rhopalodia pacifica Krammer, Navicula ramosissima (C.Agardh) Cleve, and Berkeleya rutilans (Trentepohl ex Roth) Grunow. While the spatial variation may reflect unmeasured environmental factors, the results suggest the possible influence of invasive Caulerpa algae affecting the habitat through competition with native algae. This study improves the understanding of diatom diversity in challenging environments at both regional and broader scales.
A diatom community similar to the epilithic diatom in a study of Car et al. (2019b) has also been reported for various hard substrates in the Adriatic Sea (Munda 2005, Totti et al. 2007). In a study examining seasonal fouling of artificial substrates by diatoms in the heavily polluted and eutrophic area near Piran in the Gulf of Trieste, with concrete plates (50 × 50 cm) used to observe the changes in diatom assemblages, Munda (2005) reported that the genera Berkeleya, Navicula and Licmophora dominated and covered most of the experimental surfaces. Achnanthes taxa were among the initial colonizers, while those belonging to Nitzschia and some epipelic taxa settled in autumn. Regarding the depth distribution, the highest taxa richness was observed in spring at 3 m depth and in autumn at 7 m depth (Munda 2005).
Additionally, the community structure of epilithic diatoms reported in Car et al. (2019b) study is in agreement with the results from the Northern Adriatic Sea (Ancona region) in the study by Totti et al. (2007), which was conducted on artificial hard substrates (marble, quartzite and slate) to evaluate substrate-dependent differences in colonisation and to characterise the seasonal variation of microepilithic communities in terms of abundance, biomass and community structure from April 2003 to April 2004 on a seasonal basis, and showed a dominance of motile taxa (Navicula spp., Nitzchia spp.), followed by erect (Grammatophora marina, G. oceanica,), adnate (Halamphora coffeiformis and C. scutellum) and tube-dwelling diatoms (B. rutilans). However, no significant differences in abundance and biomass were observed in the three substrates. Although Totti et al. (2007) reported high seasonal variability in epilithic microalgal communities, with lower cell densities in winter, the Car et al. (2019b) data suggested that the variation in community structure in terms of relative abundances of diatom taxa was related to sampling sites and possibly to the presence of the two invasive macroalgae considered, rather than to seasonal effects.
In addition, studies on benthic diatoms in Neum Bay (Bosnia and Herzegovina) in 2010 and 2011 (Hafner et al. 2018a, b) contributed significantly to the knowledge of diatoms in the southeastern Adriatic. The study by Hafner et al. (2018a) aimed to expand the knowledge of the MPB in the Adriatic Sea by investigating, for the first time the benthic diatom communities in the small semi-enclosed oligotrophic Neum Bay (Bosnia and Herzegovina), where benthic diatoms growing naturally on rocks and macroalgae were identified at a single station in 2010 and 2011. A total of 24 samples were taken at two different depths, 0.5 m and 8 m, and analysed by LM and SEM. A total of 425 pennate and 58 centric taxa (species and infraspecific taxa) belonging to 60 families and 115 genera were identified. The genera with the largest number of taxa were: Mastogloia (46 taxa), Navicula (36), Diploneis (35), Nitzschia (34), Amphora (31), Cocconeis (27), Achnanthes (14), Halamphora (12), Lyrella (11), and Surirella and Licmophora (10 each). Amphora bigibba var. interrupta and Cocconeis scutellum were the most common taxa, occurring in 87.5% of the samples. A total of 142 taxa were found only once (sporadic taxa). Although the benthic diatom richness in the bay was high, the taxa were not evenly distributed in time. Consistent quantitative and qualitative data are still needed to better determine the seasonal and spatial changes of the epilithic assemblages in the region.
Two recent studies were conducted to understand the development of diatom communities in relation to physico-chemical parameters (Hafner et al. 2018b, Car et al. 2020). In the study by Hafner et al. (2018b) in Neum Bay (Bosnia and Herzegovina), the taxonomic composition and structure of a marine epilithic diatom community was sampled from the bottom of the two sites at monthly intervals from January to December 2011. While the complete list of species and infraspecific taxa of benthic diatoms in Neum Bay from 2010 and 2011 (Hafner et al. 2018a) included 483 diatom taxa within 115 genera, the list of marine benthic diatoms found in Neum Bay in 2011 included 264 taxa (species and infraspecific taxa) belonging to 69 genera (Hafner et al. 2018b). Of these, 149 and 203 taxa occurred in the samples from shallow (0.5 m depth) and deep (8 m depth) sites, respectively. The monthly distribution of most diatoms was irregular, and many sporadic taxa were found. The difference between the shallow and the deep sites was mainly due to the diatom taxa that were frequently found and whose percentage abundance was high. These were Halamphora coffeiformis, Caloneis excentrica, Cocconeis scutellum var. scutellum, Licmophora flabellata, Licmophora gracilis, Licmophora sp., Navicula abunda, Rhabdonema adriaticum, and Striatella unipunctata. Canonical correspondence analysis (CCA) showed that temperature, oxygen saturation (O2/O2'), silicate concentration (SiO4) and salinity were the most important factors influencing diatom community structure in the bay.
While recent studies have focused on benthic diatoms from natural substrates in the Middle and Southern Adriatic (Car et al, 2012, 2019a, b, 2021; Hafner et al. 2018a, b, Kanjer et al. 2019, Seveno et al. 2024), some earlier studies on benthic diatoms in the Adriatic Sea were conducted on artificial substrates (e.g. Tolomio and Andreoli 1989, Tolomio et al. 1991, Bartole et al. 1991-1994, Burić et al. 2004, Munda 2005, Totti et al. 2007, Caput Mihalić et al. 2008, Mejdandžić et al. 2015). Mejdandžić et al. (2015) investigated biofilm formation on an artificial substrate made of Plexiglas (polymer of methyl methacrylate) in the Northern Adriatic Sea (Rovinj). The study, conducted in autumn 2013, at a depth of 5 m above the sea floor, analyzed diatom and bacterial succession over a one-month exposure. Samples for algological analysis were taken after 1 hour, half a day, a day or a few days, a week to one month. Selective agar plates, epifluorescence, LM, electron microscopy (EM), and high-performance liquid chromatography (HPLC) were used for pigment analysis. During the exposure, all biofilm components increased in abundance; the bacterial community was dominated by heterotrophic marine bacteria with 1.96 ± 0.79 × 104 colony forming units (CFU) cm-2, the phototrophic component was dominated by diatoms (6.10 × 105 cells cm-2), and fucoxanthin was the dominant pigment (up to 110 ng cm-2). The diatom community, led by Cylindrotheca closterium (Ehrenberg) Reimann & J.C.Lewin and other pennate benthic diatoms, comprised 30 different taxa, as revealed by detailed EM analysis. The study confirmed that a Plexiglas surface in a marine environment was susceptible to biofouling within 30 days of contact. Bacteria and cyanobacteria were initially involved in the colonisation processes, followed by diatoms, which formed a primary biofilm in the sea.
The use of artificial substrates in diatom studies offers advantages such as reduced effort and cost for sampling and processing, less habitat disturbance and higher sampling accuracy (Lamberti and Resh 1985, Lane et al. 2003). The main advantage of using artificial substrates over sampling natural habitats is the consistent standardisation between replicates. In addition, the use of artificial substrates for monitoring purposes has no impact on algal colonisation and allows global applicability without restrictions due to the natural life cycle and distribution range of macroalgae (Carreira-Flores et al. 2020, Natali et al. 2023). Although artificial substrates have been used for diatom studies for almost 100 years (Naumann 1915; cited in Tuchman and Stevenson 1980, Hoagland et al. 1986, Barbiero 2000), there is still concern about whether diatom communities that develop on artificial substrates accurately represent communities that develop on natural substrates (Lane et al. 2003). Ideally, artificial substrates should have community composition and abundance representative of natural substrates at the same site (Tuchman and Stevenson 1980, Lamberti and Resh 1985, Lane et al. 2003). But usually, the colonization patterns of artificial substrates differ from those observed on natural substrates (Mejdandžić et al. 2015). For example, diatom communities that develop on artificial substrates may better represent the diatom community of a particular natural substrate (Lane et al. 2003). Therefore, further comparative studies of diatom community structure on different artificial and natural substrates are needed.
Since 2015, only two studies, conducted by Nenadović et al. (2015) in the Middle Adriatic, and Car et al. (2021) in the Southern Adriatic, have explored the differences in composition between artificial and natural substrates. The study by Nenadović et al. (2015) focused on the initial colonization of diatoms in the periphytic community on various submerged artificial substrates, characterized by diverse physico-chemical properties, was situated in the coastal region of the Middle Adriatic Sea (Zadar region). The study analysed the abundance and genus composition of diatoms in newly formed biofilms. Additionally, comparisons were made with the diatom community on a natural substrate, specifically the seagrass P. oceanica sampled from a nearby meadow, to evaluate differences in abundance and composition on artificial substrates compared to an already established community on a natural substrate. This study aimed to determine the differences in abundance and composition of the diatom community on diverse artificial substrates and to discuss these differences by analysing the affinity of diatoms for specific artificial materials. Comparative investigations indicate that, while living organisms (macrophytes) and organic materials (wood, leaves) serve as supplementary nutrient sources for attached communities, the introduction of newly deployed inorganic artificial substrates (such as glass and plastic) into marine environments provides an opportunity to study the initial development and the succession of diatoms in periphyton communities (Nenadović et al. 2015). Furthermore, although Mejdandžić et al. (2015) and Nenadović et al. (2015) studied the development of periphytic diatoms on various artificial substrates (plexiglass, asbestos, painted iron, wood, concrete, glass, plastic, etc.), their results referred mostly to the generic level. The relevance of studying diatom affinity as an important fouling community on different artificial materials is to gain a comprehensive understanding of the impact of marine debris. This understanding is essential for systematic work on alleviating the negative impacts of litter on the marine environment.
In addition, to understand the differences in composition on artificial and natural substrates, three habitats (epilithon, epiphyton and artificial substrate) were compared in a recent diatom study by Car et al. (2021). Weekly samples were taken in August and September 2016 from the marine lake Mrtvo More in the Southern Adriatic. The study involved detailed LM and, for the first time, SEM analyses of benthic diatoms from a marine lake. A total of 97 taxa within 42 genera were identified, with Cocconeis scutellum and Halamphora coffeiformis being the most frequent. Shannon-Wiener diversity index (H') values varied from 1.78 to 4.52 throughout the year. Two groups were recognized (1): epilithon and artificial glass substrate, (2) macroalgae. The analysis indicated that diatom communities on artificial substrates corresponded exactly to those on rock substrates, suggesting their utility as representative alternatives for studying epilithic diatoms in future experiments.
The influence of physico-chemical parameters on the initial colonisation of bacteria and diatoms on a submerged artificial substrate and the development of diatom communities was studied weekly from April to October 2016 at a station in the marine lake Mrtvo More in southern Croatia (Car et al. 2020). The physico-chemical parameters (temperature, salinity, oxygen saturation, Chl-a concentration, nutrients: silicate, phosphate, nitrate, nitrite, ammonium) varied significantly according to month and season. According to the TRIX trophic index, the lake showed different trophic characteristics: (i) oligotrophic (at the beginning and end of the study), (ii) mesotrophic (end of June to mid-July), (iii) eutrophic (end of July to mid-September). Heterotrophic bacteria peaked (69,268 cells cm-2) in early June when the diatom abundance began to increase. Among the diatoms, adnate forms were the primary colonizers, particularly Cocconeis dirupta W.Gregory var. flexella (Janisch and Rabenhorst) Grunow and Cocconeis scutellum Ehrenberg var. scutellum, while motile taxa joined the fouling communities from July to September. The lake has a high diatom species richness, with the species diversity index being highest in August. The total number of diatom taxa (285 within 72 genera detected on an artificial glass substrate is comparable to some studies of epilithic diatoms in the Southern Adriatic (Hafner et al. 2018b, Car et al. 2019b), but higher than in previous studies of periphytic diatoms growing on artificial substrates in the Northern Adriatic (Mejdandžić et al. 2015, Nenadović et al. 2015) or in a study of the surface sediment layer in the Venice Lagoon (Facca and Sfriso 2007). However, we believe that this could be due, at least in part, to differences in the methodology used. This study has shown that, among the physico-chemical parameters, temperature, salinity and nitrate concentration have the greatest influence on the abundance of diatom species and that species diversity increases with nutrient enrichment. Strong correlations between environmental variables and diatoms were found and shifts in dominance at the species level were observed.
Two of the most recent studies on benthic diatoms in the Adriatic Sea (Seveno et al. 2023, 2024) focused on blue Haslea species, known for the synthesis of blue-green water-soluble pigments such as marennine, which have allelopathic, antioxidant, antiviral and antibacterial properties. Recent descriptions of new species (H. karadagensis, H. nusantara, H. provincialis and H. silbo) have deepened our understanding of these pigments. While their beneficial properties have been demonstrated under laboratory conditions, the dynamics of blooms of blue Haslea spp. in its natural habitat have been limited to oyster ponds. The study by Seveno et al. (2023) is the first documentation of benthic blooms of Haslea spp. in open environments, particularly on periphyton covering turf and macroalgae-like Padina. Two blue Haslea species - H. ostrearia and H. provincialis — were recorded at monitoring sites in the Mediterranean (Corsica, France and Croatia). The blooms followed the spring phytoplankton bloom and occurred in shallow, calm waters, suggesting that light plays an important role in their formation. The end of the blooms, marked by a warming of the upper water masses, coincided with shifts in the biofilm community, highlighting the importance of environmental conditions in regulating these unique benthic phenomena (Seveno et al. 2023).
Most work on diatoms in the Adriatic Sea is based on LM and EM, while molecular biology techniques are only sporadically used to analyse benthic diatom samples (Lobban et al. 2015, Witkowski et al. 2016, Li et al. 2019, Baldassarre et al., 2023). New molecular techniques can expand our knowledge of a species and enhance the understanding of its morphological range, biogeography and reproductive isolation (Medlin 2018). DNA sequencing has had a significant impact on the phylogeny, evolution and systematics of diatoms at the species and population level (Medlin and Kaczmarska 2004, Sorhannus 2007, Souffreau et al. 2011, Theriot et al. 2010, Witkowski et al. 2016). DNA metabarcoding has established itself as an alternative to LM-based identifications due to its speed, reproducibility and cost (Zimmermann et al. 2015). However, it is well known that a species can produce different diatom morphologies depending on the season or habitat (see references in Cox 2014), and it has been proposed that the taxonomic level of forma be used to reflect morphologies that change with specific environmental conditions (Cox 2014, Medlin 2018). Morphological changes are particularly striking in pennate diatoms, but they may be more subtle in centric species (Medlin 2018). Conflicts may arise between phylogenetic/molecular speciation and morphological speciation. It is recommended that a combination of species-level methods (both molecular and traditional) be used to resolve the species boundary issues. It cannot be assumed that the barcode of a single individual of a species is representative of the species, as different individuals in a population and individuals in different geographic populations may have slightly different barcode sequences (Medlin 2018). To get a semi-quantitative overview of the diversity of an environmental sample, we would theoretically first need to know the barcodes of all possible organisms in the biosphere. Unfortunately, we are still very far away from such knowledge and it is questionable whether we will ever know them. GenBank provides sequences for perhaps five hundred identified species of marine and freshwater diatoms (out of an estimated 100,000 species). The problem is that these sequences are underpinned with few voucher images and environmental data. A larger dataset of voucher sequences would improve DNA barcoding and allow easier and faster identification of diatoms. This is otherwise a time-consuming process, requiring diatom taxonomists to spend considerable time identifying and characterizing assemblages under the microscope. Currently, the traditional LM method for identifying diatoms in a mixed sample is still faster and more reliable than DNA barcoding of environmental samples for an experienced diatom researcher (Jahn et al. 2007). However, the combination of LM and DNA approaches provides stronger support for ecological studies of benthic microalgal communities in shallow coastal environments than either approach alone (Pérez-Burillo et al. 2022). In the study on the impact of MOSE (Experimental Electromechanical Module) flood barriers on the microphytobenthic community of the Venice Lagoon in 2019 and 2020, the integration of classical taxonomy with 18S rRNA gene metabarcoding enabled a more comprehensive understanding of the potential of the whole community and underlines the complementarity of these methods in ecological research (Baldassarre et al. 2023).
Generally, SEM has revolutionised the systematics of diatoms by revealing ultrastructural features visible that are invisible to LM, becoming essential for the delimitation of taxa; it is an essential aid to diatom classification (Round et al. 1990). The introduction of multivariate statistical methods has further advanced diatom studies by revealing complex morphometric features. More recently, molecular biology techniques have provided even more insight into variation and greatly facilitated species discovery and delimitation (Alverson 2008). Overall, these tools have improved our ability to recognise subtle differences. In addition, collections support the ongoing discovery, classification and revision of diatom species and genera, contributing to the complex and dynamic taxonomy of diatoms. The first institutional collection of permanent microscopic specimens of diatoms in Croatia, the Croatian National Diatom Collection, was established in March 2018 at the University of Zagreb, Faculty of Science. The collection contains, among other things, type specimens of newly discovered species in Croatia, which are no longer sent to other European collections, but are kept in Croatia as national biodiversity treasures. Collections such as this represent an invaluable wealth of information for biological research, providing insights into the diversity of diatom taxonomy, the biodiversity of specific areas, species distribution and temporal and spatial changes. Taken together, this information facilitates the effective protection of water ecosystems in Croatia (Gligora Udovič and Ljubešić 2021).
Since studies on the distribution of benthic diatom species along the Adriatic coast are scattered in space and time and provide rather heterogeneous information in terms of methodology, sample size and organisms studied, this review aims to provide an updated and integrated picture of Mediterranean benthic diatoms in the coastal waters of the Adriatic Sea, based on the studies conducted over the last 35 years. This paper aimed to compile a list of benthic diatom species detected in the Adriatic coasts based on the results of the most important studies carried out so far on marine diatoms and includes an updated nomenclature. The list was created to consolidate all previously scattered data for future research on benthic diatoms in Croatia, other coastal areas and beyond. This list aims to expand the knowledge of marine diatoms in the Adriatic Sea by reviewing previous studies and including an updated nomenclature.
Methodological approach
This study is based on literature records of marine diatoms recorded in the Adriatic Sea. The literature search resulted in 52 references from 1989 to 2024 (On-line Suppl. Tab. 1), including planktonic diatoms and marine benthic diatoms in the coasts, coastal lakes and lagoons of the Adriatic Sea. References included articles, reviews, checklists, and PhD theses.
The diatom checklist was compiled using information from publications dating from 1999 to 2021. The old published data of diatoms from the Adriatic Sea by Viličić et al. (2002) were supplemented with more recent information. The main sources for the diatom checklist, besides Viličić et al. (2002), are Sdrigotti et al. 1999, Cibic et al. 2007a, Hafner et al. (2018a, b), Rogelja et al. 2018, and Car et al. (2019b, 2020, 2021).
The results of this study depend on the correct identification by the authors of each reference. In most cases, there are no photographs or illustrations of the taxa, so verification of the records is difficult and some identifications may be incorrect. Despite efforts to avoid synonyms or the changed names of longer established taxa, some taxa may be listed here twice under different names.
For each species, information on its study areas was provided (Tab. 1, Fig. 1).
Tab. 1. Checklist of diatoms found in the Northern (NA), Middle (MA) and Southern (SA) Adriatic Sea.
The freshwater species reported in some studies were not excluded due to river pressure and altered salinity at different sites.
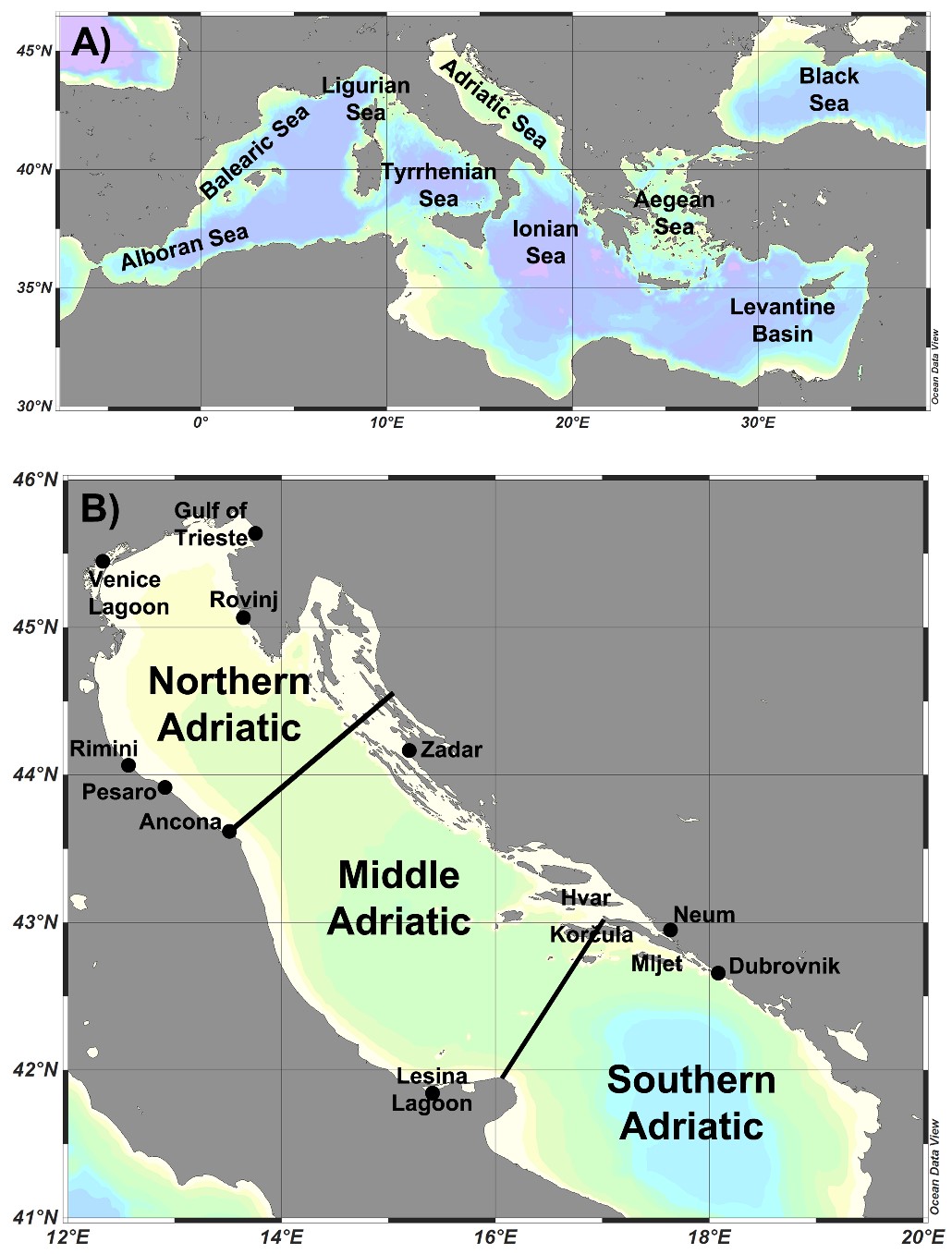
Figure 1. A) Major seas of the Mediterranean, B) Locations of the studies of diatom taxa from the Adriatic Sea presented in Suppl. Tab. 1.
Taxonomic classification was done according to Round et al. (1990). Previously, some species were given names that differed from those of the latest taxonomic order. An attempt was made to classify the species with updated current names according to Guiry and Guiry (2024), Kociolek et al. (2024) and the website Algaebase (http://www. algaebase.org/), which is constantly updated and therefore has a different taxonomic hierarchy than the one previously used.
The current status of marine benthic diatoms in Adriatic
The present work represents the first detailed list of marine benthic diatoms in the Adriatic Sea. An overview of all 52 publications included in this review shows that diatoms from the Northern Adriatic were analysed in most studies (N=32), followed by Middle Adriatic (N=15) and Southern Adriatic (N=12). Only two studies analysed diatoms on a larger spatial scale covering all regions of the Adriatic (On-line Suppl. Tab. 1). The literature was unevenly distributed, with 30 papers for the Italian coast and 18 for the Croatian coast. The coast of Bosnia and Herzegovina, on the other hand, has been the subject of 2 studies, while Slovenia and Albania are represented by 1 study each (On-line Suppl. Tab. 1). The number of publications on diatom research fluctuated, with the most productive period being that of 2000-2024, during which 47 papers were published (On-line Suppl. Tab. 1). In certain periods, research activity has increased considerably in particular areas, such as the recent intensive studies of diatoms in the Southern Adriatic. Since the beginning of diatom studies mostly in Northern Adriatic, several authors have made significant contributions to the composition of diatoms over the years.
In this paper, we merge the data with the most recent nomenclature, focusing on the current names of the cited taxa (On-line Suppl Tab. 2). A list of updated species nomenclature (On-line Suppl Tab. 2) cited in the previous studies (Cited Name) was given (Tab. 1) and the species/genus names were checked for validity using AlgaeBase (Guiry and Guiry, 2024).
Marine diatoms were represented by 822 species (163 genera and 70 families) (Tab. 1). The number of pennate species represented was 719. Families with the highest number of genera were: Naviculaceae (8), Bacillariaceae (7), Surirellaceae (6), Fragilariaceae (6). Additionally, the highest number of species and infraspecific taxa belonged to the Naviculaceae (95) and followed by Bacillariaceae (88). The taxonomic composition of benthic diatoms in the Adriatic may be defined as the community characterized by genera such as; Mastogloia (65 taxa), Navicula (62), Nitzschia (53), Amphora (52), Diploneis (43), Cocconeis (37), Halamphora (24), Achnanthes (22), Licmophora (22), Tryblionella (19), Fallacia (15), Surirella (14), Grammatophora (13), Lyrella (12).
Altogether, 381 taxa were found in the Northern, 524 taxa in the Middle and 373 taxa in the Southern Adriatic Sea. Among the total 822 diatom taxa, 177 taxa were found exclusively in the Northern Adriatic, 195 were found exclusively in the Middle and 94 occurred only in Southern Adriatic (Tab. 1). The studies used in this checklist have shown that many species were documented as first records in the Adriatic.
The variation in findings of benthic diatoms across different geographical areas may also be attributed to differing research methodologies. In the Adriatic Sea, benthic diatom analyses are often conducted within the framework of other research studies, such as phytoplankton investigations. Additionally, studies of benthic diatoms make use of various substrates, both natural and artificial.
However, the number of diatom species on the Adriatic coasts is similar to the checklist of marine diatoms from Turkish coastal waters (Kaleli and Akçaalan 2021), and the reason for the low number of marine diatoms (767 taxa belonging to 183 genera, of which 567 taxa belonged to Bacillariophyceae in Türkiye) may be attributed to the restricted scope of benthic studies along these coastlines, as highlighted by Kaleli and Akçaalan (2021). However, recent findings (Kaleli et al. 2020) suggest a potential increase in the number of taxa in forthcoming studies for both the Turkish coastal waters and the Adriatic Sea, particularly with those specifically geared towards the detection of benthic species.
This paper revealed the benthic diatom composition in the Adriatic coastal waters with the latest nomenclature and could be used as a fundamental list for later studies.
Conclusion
The study of marine diatoms on the coasts of the Adriatic Sea began more than 100 years ago, and in the last 35 years, extensive studies have been carried out. However, many geographical areas have not yet been studied and their diatom composition remains unknown. Therefore, this checklist aims to give an overview of the benthic diatoms that have been studied in the last 35 years and to create a database with updated nomenclature to fill the gap regarding marine benthic diatoms along the Adriatic coasts.
So far, however, there has been very little information on benthic diatoms in the Adriatic Sea. The knowledge of benthic diatom communities in the Adriatic Sea is poor compared to longer-term studies on phytoplankton. In this work, studies on marine plankton and benthos were combined to create a dataset that could be comparable for future studies in the Adriatic and other regions. Although checklists existed for marine plankton, this study provided a list of diatoms that included both forms from the Adriatic coasts. The inclusion of data on benthic taxa improves the knowledge of overall microalgal diversity and of coastal and transitional ecosystem ecology. The list included many benthic diatoms as well as the planktonic forms mentioned above but showed that not all the taxa on the Adriatic coasts are fully covered. Future studies should focus on the composition of benthic diatoms using LM and EM to provide additional data for further implications. Morphological details of benthic diatoms would not only increase the knowledge of taxonomy but also of species diversity and geographical distribution. However, as many of the species were not illustrated, future studies may increase the knowledge of accurate identification based on illustrated studies in the region. It should be noted that there have been no changes in the taxonomy of genera and species. The nomenclature has been updated for several taxa that have been transferred or are currently no longer in use. Therefore, further studies should be carried out to clarify the problems of diatom systematics in the Adriatic coastal waters.
This is the first exhaustive checklist of benthic diatoms of the Adriatic Sea, including the latest papers, updated with very recent books and websites, and can be used as a valuable tool for further studies. However, it should be noted that the checklist should be updated over time. Further studies on the structure of the diatom flora would provide additional information that would lead to a better ecological understanding and an approach to coastal ecosystem monitoring to prevent damage to marine biodiversity, especially for the disturbed Adriatic coasts. Nevertheless, knowledge of the composition and spatial distribution of marine benthic diatoms along the coast of the Adriatic coast remains limited.
Acknowledgements
We would like to dedicate this review to the late Professor Andrzej Witkowski, an esteemed and passionate diatom taxonomist. We will never forget Andrzej’s enthusiasm, energy, kindness and immense diatom knowledge, which combined historic and modern perspectives and left an indelible mark worldwide. We will remember him as a noble and kind person who was extremely active internationally and whose network spanned numerous countries on most continents.
This study is supported by the Croatian Science Foundation under project numbers IP-2014-09-2945 (AdMedPlan), IP-2019-04-9043 (DiVMAd), and UIP 2020-02-7868 (Adri LIFE), and by the Scientific Research Projects Coordination Unit of Istanbul University (Grant number: MAB-2021-37652). The authors thank the researchers who provided literature for this study. This checklist was made possible by the cooperation of many colleagues who provided less accessible literature. We thank the reviewers for their comments, which greatly improved this study.
