1. Introduction
Fish, more importantly, fatty fish, contains a variety of essential nutrients such as omega-3 polyunsaturated fatty acids, protein, minerals, vitamins, and bioactive compounds (Kundam et al., 2018), which may contribute to multiple health benefits in humans. Especially sardine ( Sardina pilchardus) is an important fish species since it is not only a cheap protein source, but is also acknowledged as a good reservoir for omega-3 fatty acids (Wawire et al., 2019; Guedes et al., 2020). Therefore, the consumption of sardine is associated with favourable health effects on both elder people and infants (Olmedo et al., 2013). In Turkey, the total capture of sardine was 21.265 tons in 2020, which encompasses the 8.24% of pelagic fishes captured (TUIK, 2021). Around the globe, seafood proteins constitute 7% of total protein intake and 17% of animal protein intake (FAO, 2020). Nevertheless, the consumption of fish is predicted to be doubled in the closer future (Vaughan 2021).
In recent years, the demand for “ready to eat” seafood has been a spotlight on meeting the need of today’s consumers (Behera et al., 2019). However, fish and seafood are susceptible to disruption after capturing due to factors such as endogenous enzymes, microbial growth, or oxidation (Olatunde and Benjakul, 2018). Considering the health benefits and the increasing consumption, it is crucial to preserve fish and seafood from deterioration (Uçar, 2020). Fundamental preservation techniques, that have been used in the processing of fish, could be aligned as smoking, salting, freezing, drying, and marination (Abraha et al., 2018). Fatty fishes such as sardine, anchovy, etc., are suited to marination due to their fat contents (Kilinc and Çaklı, 2004).
Marination is described as a process that encompasses the use of acetic acid and salt (Gokoglu and Uçak, 2020), and marinated seafood is considered a semi-preserved, ready-to-eat food product. The objective of marination is to prolong the shelf life while enhancing the quality and structural features (Pons-Sanchez et al., 2005). Fish can be marinated by three different methods: cold, boiled and fried. For fried marination, fish is fried with vegetable oil before the immersion into a marination solution formulated with acetic acid, salt, and other additives (Kılınç and Çaklı, 2004). However, the oxidation of polyunsaturated fatty acids is the main problem that could result in rancid flavour and lower nutritional value through the marination of fatty fish (Topuz et al., 2014).
Oxidative changes in seafood can be delayed by various effects of antioxidants. For this purpose, synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), are used as additives, but the adverse effects of these antioxidants made consumers prefer clean-label food products. Therefore, the use of antioxidants from natural sources has shined out as a hot research topic. Currently, natural additives such as garlic and hot pepper sauce (Fıçıcılar and Genccelep, 2020), pomegranate peel and artichoke leaf extract (Essid et al., 2020), rosemary extract (Cadun et al., 2008), lemon juice (Šimat et al., 2019), rosemary, coriander, laurel oils (Kocatepe et al., 2019), olive leaf extract (Testa et al., 2019), pomegranate and plum sauce (Korkmaz et al., 2021), bay leaf and green tea extracts (Ficicilar et al., 2018), grapefruit juice and pomegranate juice concentrate (Serdaroğlu et al., 2015) have been utilized in the marination of different fish.
Barberry ( Berberis vulgaris L.) fruit is cultivated in Asia, Europe, and the Middle East. Extracts of barberry fruit could be characterized by alkaloid compounds, including palmatine, berbamine, berberine, chlorogenic acid, caffeic acid, flavonoids, and vitamins (Sarraf et al., 2019; Rahimi-Madiseh et al., 2017). Acorn ( Quercus ilex) is a fruit belonging to the oak tree species containing carbohydrates, proteins, minerals, and vitamins A and C and is imposed upon the production of bread and jelly in many countries (Korus et al., 2015). Phenolic compounds, such as tocopherols, flavonoids, tannins, gallic acid, ellagic acid, different galloyl, hexahydroxydiphenyl derivative, and tannins, are considered to be bioactive compounds in acorn extracts (Akcan et al., 2017). Being a rich source of different alkaloids and phenolic compounds, both barberry and acorn extracts could be able to delay oxidative changes in seafood formulations. Notwithstanding this situation, both barberry and acorn extracts have high antioxidant potentials. As far as we know, no study has investigated the effects of using barberry and acorn extracts in marinade formulations. Hence, this study was designed to examine the impact of barberry or acorn extracts on the quality parameters of fried sardine marinades.
2. Materials and methods
Materials
Fresh sardines ( Sardina pilchardus), each approximately 55–70 g in weight and 12–14 cm in length, were procured from the local market of Izmir (Turkey). Sixteen kg of fresh, daily caught sardines were used in this experiment. Sardines were transported to the laboratory without breaking the cold chain in an icebox. Before the marination process, fish was beheaded, gutted, washed and filleted. Then fillets were immersed in a 5% salt solution for 5 min. In all marinade solutions, 4% salt, 1% grape vinegar, and 0.2% potassium sorbate, were used. Sunflower oil (Küçükbay Oil and Detergent Industry Inc., Izmir, Turkey) was used in the frying process. Grape vinegar (Carl Kühne Fermentation and Food Industry and Trade Inc., Izmir, Turkey, 6% acetic acid), salt (Refıne Billur Salt Industry Inc., Izmir, Turkey), potassium sorbate (Kimbiotek Chemical Substances Industry and Trade Inc., Istanbul,Turkey), barberry (Zencefil Organic Cosmetics and Food Industry and Trade Limited Company, Ankara, Turkey) and acorn (Doğalsan Agriculture, Ankara, Turkey) were used for the marination process.
Preparation of barberry and acorn extracts
Extraction of bioactive compounds from acorn ( Quercus ilex) and barberry fruits ( Berberis vulgaris) was carried out according to Görgüç et al. (2020) and Yılmaz et al. (2019), respectively, with some modifications (Figure 1). Fresh acorns were cleaned from foreign materials and dried in a tray drier at 50 ℃ (approximately 8% moisture content). Barberry fruits were supplied in dried form. Dried and grounded acorn powder was sieved (0.35 mm) to obtain a homogeneous particle size before the extraction process. Acorn powder (at a ratio of 1:5 w/v) or dried barberry fruit (1:10 w/v) were mixed with ethanol (96%) and the mixtures were placed in a microwave extractor (Sineo, MAS-II Plus, China) and the extraction process was carried out at 45 °C for 30 min. Subsequently, the acorn: ethanol mixture was put into the shaking water bath (Daihan Scientific, Maxturdy-30, Korea) operating at 45 °C and 65 rpm, for 30 min., to obtain the superior extraction. Lastly, both mixtures were filtered and the solvent was removed by using a rotary evaporator (Buchi R300 Rotary Evaporator, Germany).
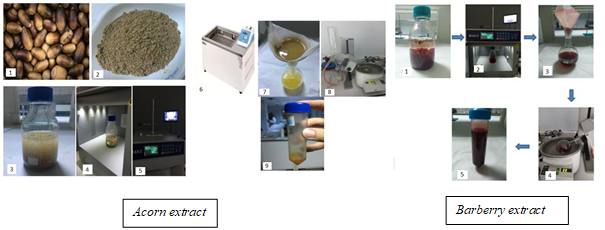
Figure 1. Preparation of acorn and barberry extracts
Marination process
Sunflower oil (200 ml), pre-heated (175 °C ) in a granite pan (diameter 26 cm, height 7 cm, Karaca, Turkey), was used for thefrying process (200 mL), and sardine fillets were cooked for 3 min. (1.5 min. each side). The marination of fried sardine fillets was carried out in a marinade solution containing 4% salt, 1% vinegar, 0.2% potassium sorbate (C) or 200 ppm BHT (B), 200 ppm gallic acid equivalent of barberry extract (BE), 200 ppm gallic acid equivalent of acorn extract (AE). Four kg of sardines were used for each batch. The control (C) group only contained 4% salt, 1% grape vinegar, and 0.2% potassium sorbate. In addition to these, B was supplemented with 200 ppm BHT, BE with 3.03 g/kg red barberry extract, and AE with 6.56 g/kg oak acorn extract. The fish and solution ratio was set to 1:1.5 (w/v). The production flow chart and the experimental design of this study are presented in Figure 2. Treatments were kept in a refrigerator (+4 °C) for 28 days and analyses were carried out weekly. Five jars of each marinade formulation were prepared and analyses were carried out in triplicate.
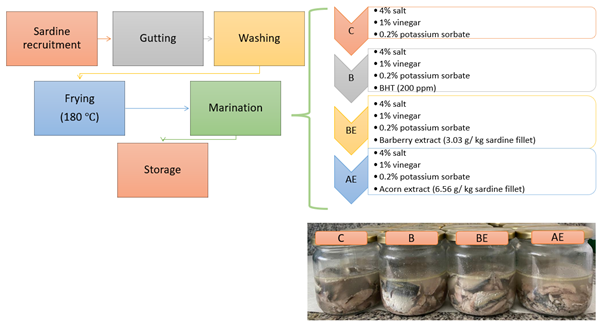
Figure 2. Production flow chard and experimental design of fried sardine marinades
Total phenolic (TP) content
The Folin-Ciocalteu (FC) method was used to determine the total phenolic contents of the barberry and acorn extracts (Singleton and Rossi, 1965). 30 μL extract and 150 μL FC reagent were sequentially transferred to test tubes containing 2.37 mL dH2O. 450 μL saturated Na2CO3 was added to the mixture (after 8 min.). After a 30-minute incubation at 40 °C, the absorbance was measured at 750 nm using a spectrophotometer (Biochrom Libra S70 UK) against a blank. The results were given in milligrams of gallic acid equivalent per gram.
Detection of bioactive compounds
The bioactive compounds of barberry and acorn extracts were determined according to Gumus et al. (2022), with some modifications, by using LC-QTOF. Chromatographic separation was performed using a HPLC Agilent 1260 Infinity series (Agilent Technologies, Santa Clara, CA, ABRE) instrument with a Poroshell 120 EC-C18 (3.0X150 mm, 2.7 μm), the mobile phase: 5 mM ammonium format in water (A) and methanol (B) using a gradient elution as follows: 0-0.5 min, 10% B; 0.5-5 min, 70% B; 5-7 min, 95% B; 7-10 min, 95% B; 10-15 min, 10% B. MS analysis was performed using an Agilent 6550 iFunnel equipped with the Agilent Dual Jet Stream electrospray ionization (drying gas flow, 14.0 L/min; nebulizer pressure, 35 psi; gas drying temperature, 290 °C; sheath gas temperature, 400 °C; sheath gas flow, nitrogen 12 L/min).
Marinade uptake for an individual treatment was calculated as a percentage of sardine's weight gain comparing with regard to the initial weight (Lombard and Lanier, 2011):
Chemical composition and pH
Moisture and ash contents of fillets were calculated according to AOAC (2012) procedures. Fat content was determined using a chloroform-methanol (cold extraction) method stated by Flynn and Bramblet (1975). The protein content of samples was determined using an automatic nitrogen analyzer (FP 528, LECO, Michigan, USA) based on Dumas’s method. pH value of fresh sardines, marinades and fried sardines were measured in quadruplicate by using a pH-meter (WTW pH 3110 set 2, Germany) equipped with a glass penetration probe.
Lipid oxidation analyses
The peroxide value (PV) was calculated using the method described by theAmerican Oil Chemists' Society (1990) and AOAC (2012). The sample was homogenized in 30 mL of chloroform and filtrated with 30 ml acetic acid and 2 ml of KI was added. After waiting for 5 min in the dark, distilled water and 2 ml of 1% starch solution (w/v) were added to the solution. Lastly, the mixture was titrated with 0.01 N sodium thiosulfate until the blue colour perished. Thiobarbituric acid reactive substances (TBARS) value is a measure of secondary lipid oxidation products as mg malonaldehyde (MA)/kg using the modified method of Witte et al. (1970). TBARS analysis was based on the measurement of the intensity of the formed pink colour (malonaldehyde) as a result of oxidation at a wavelength of 532 nm. The obtained absorbance value was multiplied by 5.2 and the malonaldehyde concentration of the sample was determined as mg MA/ kg.
Colour measurement
The lightness (L⁎), redness (a⁎ > 0), and yellowness (b⁎ > 0) parameters of marinated sardines were measured with a portable colourimeter (Chromameter CR400, Konica Minolta, Japan), using a D65 standard illuminant and a 10 standard observer (C.I.E, 1978). Three points of four marinated fillets were used for each treatment.
Trimethylamine (TMA-N) analysis
100 g of fried sardines were blended with 200 ml of 7.5% trichloroacetic acid solution and centrifugation was carried out until a clear supernatant was observed (2000 to 3000 g). 4 ml supernatant was added into a tube, a blank, and standards were prepared. 4 ml distilled water was used for the blank and for standard solutions 1.0, 2.0 and 3.0 ml of working standard solution (0.01 M TMA/ml) each, diluted to 4 ml with distilled water. 1 ml of 20 % perchloric acid, 10 ml anhydrous toluene, and 4 ml of 10% potassium carbonate solution were added to all test tubes (blank, standard, samples). Subsequently, to dry the toluene, tubes were vortexed after the addition of 0.1 g anhydrous sodium sulfate. Lastly, 5 ml 0.02% picric acid working solution was added and the content inside the tubes was mixed and absorbance recorded at 410 nm against the blank. The levels of TMA-N (mg N/100g sample) were computed as follows (Idakwo et al., 2016):
Where A = absorbance of sample, A1 = absorbance of standard nearest to absorbance of sample, Vx = mg TMA standard solution, Vt = volume (ml) of solution used, and Vs = volume (ml) of the aliquot of sample used.
Sensory analysis
Sensory analysis was implemented by 12 panelists (55% women and 45% men, 20-35 years old), according to Witting de Penna (2001). Approximately 20 g of sardine fillets coded with random three-digit numbers were served to the panelists. Panelists were colour, texture, flavour, and general acceptability by using a nine-point hedonic scale manifested by Peryam and Pilgrim (1957). The categorization of the scale was as follows: “like extremely (9); like very much (8); like (7); like slightly (6); neither like nor dislike (5); dislike slightly (4); dislike (3); dislike very much (2), and dislike extremely (1).” Water and saltless crackers were provided to the panelists to cleanse their mouths between the treatments.
Statistical analysis
Five jars (800 g fillet for each jar) of each marinade formulation were prepared and all analyzes were performed in triplicate. All the results were given as the mean ±standard deviation. The statistical analysis was conducted at a 95% confidence interval using SPSS for Windows 21.0. The effects of the use of barberry or acorn extracts on the related quality attributes were observed by one-way analysis of variance (ANOVA), while the impact of storage time on quality was determined by two-way ANOVA. Duncan's multiple comparison test was applied to detect the significant differences (P < 0.05).
3. Results and discussion
Properties of barberry and acorn extracts
Colour parameters, pH, and total phenolic contents of barberry and acorn extracts are presented in Table 1. L*, a*, and b* values of barberry extract were found as 19.07, 18.41, and 13.40, respectively. Çakır and Karabulut (2020) reported similar L*(21.91), a*(19.37), and b*(8.81) values for barberry extract. These differences in colour values are thought to be due to the growing conditions of barberry’s and extraction methods. Colour parameters for acorn extract were found as follows: L*:1.80, a*: -0.57, and b*: 2.35. Amessis-Ouchemoukh et al. (2017) stated higher L*, a*, and b* values for acorn extract than our findings. The total phenolic contents of barberry and acorn extracts were 15.23 and 33.16 mg/g in terms of gallic acid equivalent. Different research revealed various phenolic contents for both extracts. The phenolic contents of barberry extracts reported in other studies were 63.2 mg GAE/g (El-Saber Batiha et al., 2020) and 3269.05 mg GAE/kg (Jaberi et al., 2020), while acorn extract was reported to have 928 mg gallic acid in its 100 grams (Custódio et al., 2015). Differences in colour parameters and phenolic contents mostly depend on the maturity stage, harvest area and season, as well as the applied extraction method.
Table 1. Colour parameters, pH and antioxidant properties of barberry and acorn extracts
| Color |
Total phenolic content (mg GAE/g) | pH | |
|---|---|---|---|
| Barberry extract |
L*:19.07±0.65 a*:18.41±0.98 b*:13.40±0.37 | 15.23 | 2.56 |
| Acorn extract |
L*: 1.80±0.27 a*: -0.57±0.08 b*: 2.35±0.31 | 33.16 | 5.53 |
Bioactive compounds of barberry and acorn extracts
Bioactive compounds present in barberry and acorn extracts are shown in Table 2. Redha et al., (2021) stated that lupeol, oleanolic acid, stigmasterol, stigmasterol glucoside, berberamine, palmatine, berberine, oxyberberine, columbamine, isocorydine, lambertine, magniflorine, oxycanthine, N-(p-trans-Coumaroyl) tyramine, cannabisin G and (±)-lyoniresinol were isolated from Berberis vulgaris. Thirty-two different phenolic compounds were distinguished in different acorn extracts and mostly all of them were gallic acid derivatives, in the form of either galloyl esters of glucose, the combinations of galloyl and hexahydroxydiphenoyl esters of glucose, tergallagic O- or C-glucosides, or ellagic acid derivatives. Variations in plant part, solvent selection, extraction temperature, pressure, and duration can all contribute to the observed diversities in the constituent profiles of herbal extracts (Hita et al., 2021; Hahm et al., 2021).
Table 2. Bioactive compounds in barberry and acorn extracts
pH value of marinades and marination absorption
pH values of marinades were 4.73 (C), 4.67 (B), 4.53 (BE), and 4.68 (AE). The addition of different antioxidant sources significantly lowered the pH value (P < 0.05). The lowest pH value was found in BE solution formulated with barberry extract, while the control sample had the highest pH. The lowest pH value in BE marinade could probably be associated with organic acids such as ascorbic acid, malic, citric, and tartaric acids in barberry fruits (Akbulut et al., 2009; Gundogdu, 2013). Marinade solutions diffuse from the surface into the interior parts of the muscles. The marinade absorption percentages were found as 14.24%(C), 12.04% (B), 11.90% (BE), and 11.87% (AE). The addition of BHT or natural extracts was not effective to alter the liquid absorbed by the sardine muscles (P > 0.05). Rimini et al., (2014) stated that the marination of chicken meat with thyme and orange essential oil blends did not affect marination uptake (7.48-7.74% for chicken wings, 8.42-8.71% for chicken breast meat). Serdaroğlu et al., (2007) stated that marination uptake of turkey breast muscle ranged between 6.9-13.7% by using different marinade solutions (citric acid and/or grapefruit juice), with the highest uptake observed in marination with 0.1 M citric acid. In contrast to our results, marinade absorption of chicken breast muscle ranged between 37.37-64.52% by using juniper extract, where increasing amounts of juniper extract decreased the marinade absorption (Kavuşan et al., 2021). Marinade absorption by the muscle depends on the marinating technique or whether the product, which is to be marinated, is raw or cooked. Therefore, in our research, it is thought that the protein denaturation, as a result of frying, may have led to less absorption of marinade solutions.
Chemical composition
Table 3 shows the moisture, protein, fat, and ash contents of fried sardine marinades added with BHT, barberry, or acorn extracts as antioxidant sources. The addition of BHT or extracts to the marinade formulation did not affect the chemical composition (P>0.05). The moisture, protein, fat, and ash contents were found to be between 69.50-69.93%, 18.66-19.51%, 8.06-8.55%, and 2.78-2.98%, respectively. Similar to our study, there were no statistical differences in the chemical composition of shrimps marinated with rosemary extract and the control group (Cadun et al., 2008). Also, the use of bay leaf or green tea extracts in anchovy marinades was found nugatory on chemical composition (Fıçıcılar et al., 2018).
Table 3. Chemical composition of marinated sardines
Data were presented as the mean ± standard deviation.
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
pH
pH value in fishery products is an important physicochemical parameter to predict fresh fish muscle quality. pH value in fresh sardines was 6.3. Table 4 shows the effect of the additives and storage time on the pH values of marinade solutions added with BHT, barberry extract, and acorn extract. pH values of sardines decreased with the marination. However, no significant differences were observed between the treatments on the production day (initial pH). On the other hand, the influence of additives was tangible in the second half of the storage (from day 14 to 28). BE treatment had the lowest pH at each evaluation period. A probable explanation for the low pH value in BE could be sourced from the low pH of barberry extract (Table 1). Also, the incorporation of fruit extracts into pork marination lowered pH values (Kim et al., 2016). AE treatment had a similar pH value with B treatment at each evaluation period except day 14 (P > 0.05). A drastic decrease was observed in pH on the 14th day of storage. It is clear that the additives have just effectively penetrated and dispersed into the sardine fillets. At the end of the storage, the C treatment had the highest pH, while the lowest pH was measured in BE treatment (P < 0.05). The acidity of all treatments at the end of the storage was lower than the initial values except for C (P < 0.05). Similar trends were also reported by using juniper (Kavuşan et al., 2021) and rosemary extracts (İlhan 2010) in different meat products. Sardine fillets marinated with vinegar, grapefruit juice and pomegranate juice concentrate also had lower pH compared to the control treatments (Serdaroğlu et al., 2015).
Table 4. pH values of marinated sardines throughout storage
a-c Different letters in the same column indicate a significant difference (P < 0.05).x-z Different letters in the same row indicate a significant difference (P < 0.05). Data were presented as the mean ± standard deviation.
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
Peroxide values
The changes in peroxide values of marinated sardine fillets during storage are given in Table 5. Peroxide value of fresh sardines was 7.3±0.17 meqO2/kg. The highest initial peroxide value (17.81 meqO2/kg) was observed in the AE treatment, while the lowest value (13.57 meqO2/kg) was observed in the C treatment, where no antioxidant was added to the marinade formulation. There was no significant difference between the 0th and 7th day in the peroxide values of the C and BE treatments. The highly unsaturated fatty acid content of sardine muscle could be the reason for high peroxide values at the beginning of storage. No difference was observed between the other treatments and the control, except B treatment on the 14th day (P > 0.05). However, the highest peroxide value (P < 0.05) was obtained in C treatment on the 21st and 28th day of storage, as the beneficial effect of extracts added to the marinade solution. A similar result was also reported in herring meat treated with milk thistle before marination (Ochrem et al., 2021). Gökoğlu et al., (2012) and Topuz et al., (2016) demonstrated that using natural extracts resulted in lower peroxide values. The extracts of barberry and acorn showed the same antioxidant effect as BHT in later stages of storage. Hanachi and Golkho (2009) reported that the antioxidant capacity of barberry extract could be equivalent to or higher than vitamins B and E.
Table5. Peroxide values of marinated sardines throughout storage
a-c Different letters in the same column indicate a significant difference (P < 0.05).x-z Different letters in the same row indicate a significant difference (P < 0.05). Data were presented as the mean ± standard deviation.
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
TBARS
TBARS is a remarkable quality index showing lipid oxidation of unsaturated fatty acids. TBARS values were expressed in mg malonaldehyde/kg. TBARS value of fresh sardines was 0.95±0.08 mg MA/kg. The use of BHT or extracts in the marinade formulation was not found to be effective on TBARS values, except on the 14th day of storage (Table 6). Similarly, using the grape seeds, papaya seed extract, or BHT did not affect the initial TBARS values of Indian mackerel (Sofi et al., 2022). On day 14, C and AE treatments were similar and had the highest TBARS content, followed by B and BE treatments, respectively. The bioactive compounds (berberine, berbamine, ascorbic acid, chlorogenic acid, catechin, and anthocyanins) in barberry extract were thought to be the source of the observed antioxidant effect (Rahimi-Madiseh et al., 2017). Testa et al., (2019) showed that olive leaf extract was able to retard lipid oxidation, while it was not effective at the beginning of storage. Duman et al., (2015) reported that the administration of rosemary and thyme oils at doses of 300 ml/L resulted in significantly lower final TBARS values for marinated crayfish after 56 days, compared to control. In another study, where rosemary oil was used in the shrimp marination, TBARS values were found to be lower compared to the control (Cadun et al., 2008). The marination of sardine fillets by using pomegranate juice concentrate resulted in lower TBARS values (Serdaroğlu et al., 2015). Rosemary, myrtle, and nettle extracts were effective to delay lipid oxidation in anchovy fillets (Turhan et al., 2009). TBARS values tend to increase until the 14th day of storage and then they decreased (P < 0.05). The decrease in TBARS content after a peak was attributed to the interaction between malonaldehydes and protein degradation products (Reddy and Setty 1996; Fernandez et al., 1997). Final TBARS values ranged between 1.69-1.96 mg malonaldehyde/kg. It is reported that rancidity starts in fish when TBA exceeds 4 mg malonaldehyde/ kg and the limit for the product to be deemed consumable is 7-8 mg malonaldehyde/ kg (Curran et al., 1980).
Table 6. TBARS values of marinated sardines throughout storage
a-c Different letters in the same column indicate a significant difference (P < 0.05).x-z Different letters in the same row indicate a significant difference (P < 0.05). Data were presented as the mean ± standard deviation.
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
Colour
L*, a*, and b* values of sardine samples are presented in Table 7. Initial L* values ranged between 58.80-65.08, while the control group had the highest lightness on the first day of storage (P < 0.05), and other counterparts had similar L* values. Also, there was no difference between B, BE, and AE groups on day 21. However, lower L* values were obtained in the control treatment. On the last day of storage, there were no significant differences between the treatments (P > 0.05). The use of various extracts had a different effect on a* values. The lowest initial a* value was registered for AE (0.49), while B (3.07) and BE (2.67) treatments had the highest values. The lowest a* value in AE treatment could be associated with a* value of acorn extract (-0.57). The red colour of BE on days 0 and 14 may be related to the high a* value of the extract used. The utilization of green tea extract in anchovy marinade had caused darker and redder colour (Fıçıcılar et al., 2018). Despite the effects of extracts on a* values, the highest a* values were observed in the control group on the 21st and 28th day of storage, and these values decreased with the use of extracts and BHT (P < 0.05). At the storage, all treatments had a red colour compared to the initial day, except the B treatment. The use of BHT enabled redness stability throughout the storage. The b* values of the treatments varied between 9.45 (B) and 11.24 (AE). The use of natural extract resulted in higher b* values, while BHT had the opposite effect. No statistical differences were observed between the samples on the 14th and 21st day. Nonetheless, B and AE treatments had higher b* values than C and BE treatments. The darker and bluish colour was observed in herring fillets treated with elderberry marinades (Sampels et al., 2010).
Table 7. Colour parameters of marinated sardines throughout storage
a-c Different letters in the same column indicate a significant difference (P < 0.05).x-z Different letters in the same row indicate a significant difference (P < 0.05). Data were presented as the mean ± standard deviation.
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
TMA-N content
Fresh fish naturally contains trimethylamine oxide (TMAO) and TMAO is a quaternary ammonium compound responsible for osmoregulation in fish. TMA, which is responsible for ammonia-like odour and fishy odour, is formed by the microbial decomposition of TMAO and is used to determine seafood quality (Gram and Huss 1996; Serdaroğlu and Deniz 2001). TMA values of fresh sardines were 1.12 mg/100 g. The initial TMA-N contents of the fried marinades varied between 1.42-1.52 mg/100 g (Figure 3). The TMA values were higher compared to fresh sardines, but no statistical differences were found between the treatments (P > 0.05). BHT or extracts resulted in lower TMA-N values than control treatment on the 7th, 14th and 21st day of storage, regardless of the additive. Similarly, the use of olive leaves in anchovy marination was not found to be effective at the beginning. It, however, resulted in lower TMA-N content during the last stages of storage (Testa et al., 2019). Likewise, Sofi et al., (2022) observed that the utilization of grape and papaya seed extracts was effective on TMA-N content at the end of the storage. TMA contents of treatments reached to 3.14, 3.28, 4.60, and 4.98 mg/100 g for BE, AE, B, and C treatments, respectively. On the last day of storage, the lowest TMA content was observed in the BE and AE treatments (P < 0.05). The TMA-N value has been reported to be about 1 mg/100g in fresh aquaculture products and 8 mg/100 g in decayed samples (Food and Agriculture Organization of the United Nations, 1986). In addition, in terms of the quality classification of fish products with respect to the TMA-N value, the product is considered “good” with a TMA-N value up to 4 mg / 100 g, “marketable” with a TMA-N value up to 10 mg / 100 g, and “decayed” with a TMA-N value equal to or greater than 12 mg/ 100 g (Kundakçı 1989). All of the treatments were within limits of consumable quality. Only C and B treatments exceeded the “marketable level” at the end of the storage. The lowest TMA-N contents in BE and AE samples may be due to the inhibitory effect of barberry and acorn extracts on microbial growth, including TMAO-reducing microorganisms. Similarly, the use of pomegranate and artichoke extracts in sardine marinades had the lowering effect on TMA-N content (Essid et al., 2020). Also, the use of pomegranate juice (Topuz et al., 2014) and lemon juice (Topuz et al., 2016) in anchovy marination reduced the TMA-N content. TMA-N contents of all treatments increased throughout the storage. Similar results have been observed by Gökoglu et al., (2004) in marinated sardines. Significant incremental pattern in TMA-N values was observed in semi fried mullet fillets during subsequent cold storage (Yasin and Abou-Taleb 2007).
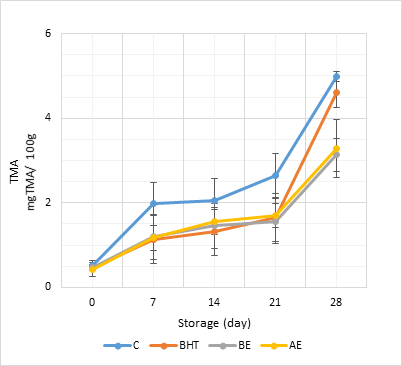
Figure 3. TMA-N contents of fried sardine marinades throughout storage
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
Sensory evaluation
The sensory evaluation results of fried sardine marinades treated with BHT, barberry, or acorn extracts are presented in Figure 4. Panelists were asked to evaluate the samples in terms of colour, surface appearance, texture, flavour, and overall acceptability. While no significant differences were observed in terms of colour, appearance, and texture at the beginning of storage, the addition of acorn extract harmed the flavour of the sample. Therefore, the flavour and general acceptance scores of the AE treatments were lower than their counterparts (P < 0.05). On the 7th day of storage, the colour scores of the C and B groups were found to be the most preferable by the panelists. However, this difference disappeared with the progress of storage. Various researchers have reported that the use of various additives darkens the colour of fish (Gökoğlu et al., 2009; Demirok et al., 2014; Essid et al., 2020). When the appearance scores were examined, no significant differences were recorded during storage. Nevertheless, the score of the AE treatment was lower than other treatments at the end of the storage. All of the samples were similar in terms of texture and all treatments received similar liking scores. The addition of extract was found to be effective in terms of flavour scores (P < 0.05). Tannins in the acorn extract caused a decrease in the flavour scores of sardine samples due to the bitter taste carried through tannins. The lowest flavour score ,and thus the lowest overall acceptability, was observed in the AE treatment throughout the storage period (P < 0.05). The use of lemon juice in the marination of anchovy fillets of more than 35% can induce undesirable sensory qualities (Topuz et al., 2016). Contrary to our study, the addition of olive leaf extract caused higher colour, flavour, texture, and general acceptance scores of marinated anchovy fillets (Testa et al., 2019).
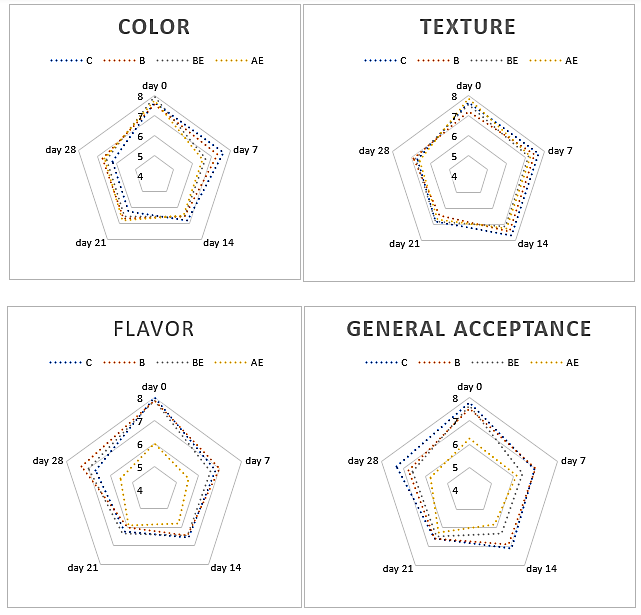
Figure 4. Sensory evaluation of fried sardine marinades
C: fried sardine marinades added no antioxidant, B: fried sardine marinades added with BHT, BE: fried sardine marinades added with barberry extract, AE: fried sardine marinades added with acorn extract
4. Conclusion
The preservation of sardines against oxidation and spoilage can be significantly enhanced by incorporating extracts derived from barberry and acorn. These natural extracts exhibit noteworthy antioxidant properties and demonstrate potential antimicrobial effects, surpassing the preservation efficacy of both the control group (lacking BHT or extract addition) and group B (marinade solution supplemented with BHT). Consequently, it can be inferred that barberry extract, in particular, represents a viable and advantageous substitute for synthetic additives traditionally employed in fish marination processes. Importantly, the utilization of barberry extract does not compromise the overall quality of the sardines. These findings underscore the potential of employing natural extracts as a safe and efficacious method for preserving fried sardine fillets, while simultaneously mitigating concerns associated with the utilization of synthetic additives. In addition to their preservation benefits, it should be noted that the use of barberry and acorn extracts in the marination process led to a more pronounced yellow colour in the sardines. Specifically, the inclusion of barberry extract resulted in a richer red colour as well. These changes in colour can be attributed to the natural pigments present in the extracts. Despite these colour variations, it is important to emphasize that the overall quality of the sardines was not negatively affected. In summary, marinating fried sardine fillets with natural extracts can serve as a safe and effective method for their preservation.
Author Contributions: Conceptualization: S. M. Formal analysis: K. H.S., Y.-B. Ö., C. H., D. İ.G., K. S. Methodology: S. M. Investigation: S. M., K. H.S., Y.-B. Ö., Writing - original draft: S. M. K. H.S., Y.-B. Ö., Writing - review & editing: S. M., K. H.S., Y.-B. Ö.
Acknowledgments: The authors would like to extend their gratitude to the Ege University for funding this study through Scientific Research Projects Coordination (Project number: FLP-2021-22400).
Conflicts of Interest: The authors declare no conflict of interest.
