INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a manifestation of multi-system metabolic dysfunction that affects the liver (1). Currently, the number of NAFLD patients accounts for 25 % of the global population, making it the most recognised chronic liver disease worldwide. The rapidly increasing prevalence of NAFLD has become a new challenge in the fields of liver disease and metabolism, posing a significant threat to public health and societal development globally (2, 3). Despite its rising prevalence, there is currently no FDA-approved treatment for NAFLD (4–6). Current management primarily focuses on lifestyle modifications, such as weight loss, as well as off-label use of drugs like pioglitazone and vitamin E. If left untreated, NAFLD can progress to liver fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) (7).
The pathophysiology of NAFLD is complex, involving the interplay of genetic, metabolic and environmental factors (8, 9). Currently, the pathogenesis of non-alcoholic steatohepatitis (NASH) mainly focuses on insulin resistance (10, 11), oxidative stress-induced damage (12, 13), inflammatory responses (14, 15), and gut microbiota dysbiosis (16, 17). Characterised by liver inflammation, lipotoxicity, oxidative stress and fibrosis, the disease is typically driven by metabolic dysregulation, including lipid metabolism imbalance and mitochondrial dysfunction (8, 9). Although NAFLD is closely associated with metabolic factors such as obesity and insulin resistance, the specific mechanisms underlying its progression, especially from steatosis to hepatitis, fibrosis and cirrhosis, remain an area requiring further investigation (18, 19).
Given the limited treatment options and the increasing burden of NAFLD, there is an urgent need for new therapeutic approaches. Among the promising candidates, astragaloside IV (AS-IV), a bioactive compound derived from Astragalus membranaceus (Fisch. ex Bunge) (Fabaceae/Leguminosae) (syn. Astragalus propinquus Schischkin) primarily cultivated in Inner Mongolia, China, and Siberian regions, has been reported to regulate immune-inflammatory factors, modulate gut microbiota, act as an antioxidant, regulate blood lipid levels, and reduce hepatic lipid deposition (20–22). Recent studies suggest that AS-IV may play a role in alleviating NAFLD by regulating lipid metabolism and gut microbiota, as well as suppressing inflammation (23–25). However, the exact mechanism through which AS-IV affects the pathological progression of NAFLD is not yet properly and fully understood.
This review aims to explore the pharmacological effects of AS-IV on NAFLD and the molecular mechanisms underlying its actions. Specifically, we will discuss the latest advances regarding AS-IV's involvement in regulating key pathological pathways associated with NAFLD, including insulin resistance, apoptosis, gut microbiota, oxidative stress and inflammation. Additionally, we will assess the potential of AS-IV as part of the therapeutic strategy for NAFLD.
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD)
Clinical features and pathological classification
NAFLD encompasses several stages of liver injury, which can be classified based on histological findings (26). Simple hepatic steatosis or non-alcoholic fatty liver (NAFL) is the early stage of NAFLD, characterised by the accumulation of triglycerides in hepatocytes without significant inflammation or hepatocellular injury (27). This stage is driven by an imbalance between lipid uptake (via CD36/FATPs), de novo lipogenesis (upregulated SREBP-1c), and impaired β-oxidation (PPAR-α suppression), creating a lipid-rich hepatic microenvironment (28, 29). This stage is typically considered benign and may be reversed through lifestyle changes such as introduction of modified diet pattern, eventual weight loss and proper exercise (27).
Additionally, NAFL can progress to NASH, defined by the presence of hepatic steatosis and inflammation, often accompanied by hepatocellular ballooning and varying degrees of fibrosis (30). Transition to NASH involves "two hits", such as mitochondrial dysfunction (ROS overproduction), ER stress (IRE1α/XBP1 activation), and inflammasome activation (NLRP3/IL-1β), which amplify hepatocyte apoptosis and Kupffer cell-driven inflammation (31–33). NASH is a more severe and progressive form of NAFLD, associated with an increased risk of liver-related complications, including cirrhosis and HCC (34). The degree of fibrosis in NASH is a key factor in determining disease progression and prognosis, with advanced fibrosis associated with significantly higher mortality and liver-related morbidity.
The final stage of NAFLD involves progression from NASH to cirrhosis, which, in some cases, may also include liver failure and HCC (35). Cirrhosis is characterised by extensive liver scarring that disrupts the normal liver architecture and impairs liver function. Fibrotic septa in cirrhosis distort hepatic vasculature, leading to portal hypertension and collateral circulation, while regenerative nodules reflect aberrant hepatocyte proliferation driven by Wnt/β-catenin signalling (36). NAFLD is the leading cause of liver transplantation worldwide, especially among patients with advanced NASH (34). As the disease progresses, patients may also experience extrahepatic complications, including cardiovascular diseases, which are often associated with metabolic dysfunction and insulin resistance (Table I) (35).
Table I. Clinical stages of non-alcoholic fatty liver disease (NAFLD) and histological features
Pathological mechanisms of NAFLD progression
The transition from simple hepatic steatosis to NASH involves complex pathological processes, including oxidative stress, inflammation, lipotoxicity and mitochondrial dysfunction (37). In NASH patients, the accumulation of fat in hepatocytes triggers an inflammatory response, leading to hepatocellular injury, apoptosis, and the activation of hepatic stellate cells (HSCs) (38). Upon activation, HSCs can transform into myofibroblasts, which secrete large amounts of extracellular matrix (ECM) proteins, such as collagen and fibronectin. The accumulation of these proteins leads to liver fibrosis (37, 38). This process is closely related to oxidative stress, inflammatory responses, and metabolic dysregulation, particularly in NASH, where the accumulation of fat and cellular damage in the liver activates the immune system, promoting HSC activation and the progression of fibrosis (39). Non-invasive biomarkers and imaging techniques, such as transient elastography and MRI-based methods, are increasingly being used to assess the severity of fibrosis and predict disease progression (40, 41).
In summary, NAFLD is a progressive liver disease, beginning with simple fat accumulation in the liver and potentially evolving into more severe conditions such as NASH, fibrosis, cirrhosis, and ultimately leading to liver cancer. The progression of the disease is influenced by multiple factors, including insulin resistance, gut microbiota dysbiosis, metabolic abnormalities and genetic susceptibility. Early identification of high-risk patients and timely intervention are critical for preventing the progression of NAFLD.
PHARMACOLOGICAL EFFECTS AND MOLECULAR MECHANISMS OF AS-IV IN NAFLD
AS-IV is a cycloartane-type saponin extracted from the roots of Astragalus membranaceus (Fisch. ex Bunge), a leguminous plant indigenous to temperate Northeast Asia, including Inner Mongolia and Siberia, a plant widely used in traditional Chinese medicine (42). AS-IV is a triterpenoid saponin with a unique cycloartane structure, composed of a glycoside structure where the sugar portion is attached to the triterpene skeleton (43). This structural feature is crucial for its bioactivity, allowing it to interact with cell membranes and regulate various cellular pathways (42, 43) (Fig. 1). To date, numerous studies using cell and animal models have shown that AS-IV has effective protective effects on the cardiovascular (44), pulmonary (45), hepatic (46), renal (47) and brain (48) systems. Additionally, AS-IV has demonstrated antiproliferative/anticancer potential by inducing apoptosis, inhibiting tumour growth, and preventing metastasis in various cancer types (45, 49). These multifaceted effects make AS-IV a promising candidate for the treatment of various chronic diseases, including liver diseases, and provide a foundation for exploring its potential therapeutic role in NAFLD.
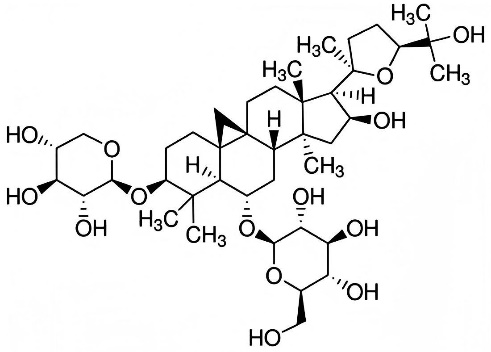
Fig. 1. The chemical structure of astragaloside IV.
Improvement of insulin resistance
Insulin resistance (IR) is a risk factor for NAFLD, characterised by the accumulation of lipids in hepatocytes (50). One of the mainstream theories explaining the complex pathogenesis of NAFLD, the "two-hit" hypothesis, posits that the "first hit" is caused by IR, which induces peripheral lipolysis and elevated insulin levels (51). The increased free fatty acids (FFAs) in the periphery lead to enhanced hepatic uptake of FFAs, while hyperinsulinemia promotes glycolysis, increases fatty acid synthesis, and ultimately results in hepatic lipid accumulation. Medium- and long-chain fatty acids are primarily oxidised in the mitochondria, whereas very long-chain fatty acids (VLCFAs) are almost entirely metabolised through β-oxidation in peroxisomes (52), a process that generates reactive oxygen species (ROS) such as hydrogen peroxide. Normally, the body has a well-established antioxidant mechanism to counteract ROS (51). However, in the case of hepatic lipid accumulation induced by IR, this balance is disrupted, and excess ROS triggers the onset of steatohepatitis through lipid peroxidation, cytokines and Fas ligand (FasL) activation. Fas (CD95/APO-1), a cell surface death receptor, binds to FasL, initiating caspase-dependent apoptosis (53). In NAFLD, ROS and lipid peroxidation products (e.g., malondialdehyde) upregulate FasL expression, promoting hepatocyte apoptosis and amplifying inflammatory responses (53). This apoptotic signalling exacerbates liver injury and fibrosis, creating a vicious cycle in disease progression (53). Further, lipid peroxidation leads to cell death, increased collagen synthesis, and ultimately the development of hepatic fibrosis in NAFLD (51).
AS-IV has been shown to improve insulin sensitivity by inhibiting protein tyrosine phosphatase 1b (PTP1B), a negative regulator of insulin signalling. In insulin-resistant HepG2 cells, AS-IV treatment increased glucose consumption and enhanced insulin receptor phosphorylation, suggesting that AS-IV helps restore insulin signalling pathways (54) (Fig. 2). This effect was linked to a reduction in triglyceride (TG) and cholesterol levels, common metabolic disturbances in NAFLD. Furthermore, AS-IV alleviates IR and lipid accumulation by activating AMP-activated protein kinase (AMPK) (23). AS-IV promotes AMPK phosphorylation, which in turn reduces triglyceride production, indicating its role in improving lipid metabolism. Additionally, AS-IV inhibits the translocation of sterol regulatory element-binding protein-1c (SREBP-1c) into the nucleus by inducing phosphorylation of SREBP-1c at Ser372, a critical step for regulating lipid biosynthesis in the liver. These findings collectively suggest that AS-IV could be a promising therapeutic agent for treating hepatic steatosis and improving insulin sensitivity in NAFLD.

Fig. 2. Schematic diagram illustrating the multi-target mechanisms of astragaloside IV (AS-IV) in non-alcoholic fatty liver disease (NAFLD). AS-IV exerts therapeutic effects through five major mechanisms: (i) improving insulin resistance by inhibiting PTP1B and activating the AMPK pathway, leading to decreased SREBP-1c nuclear translocation and reduced lipogenesis; (ii) alleviating oxidative stress via activation of the AMPK/Nrf2 pathway, increasing antioxidant enzymes (SOD, CAT) and GSH levels; (iii) reducing inflammation through inhibition of the TLR4/MyD88/NF-κB pathway and regulation of the AMPK/Akt/GSK-3β axis, decreasing pro-inflammatory cytokines (IL-1β, IL-6, TNF-α); (iv) suppressing hepatocyte apoptosis by modulating Bcl-2/Bax ratio, inhibiting cytochrome c release, and reducing caspase activation; (v) restoring gut microbiota balance and bile acid metabolism by increasing beneficial bacteria (Akkermansia muciniphila, Lactobacilli), reducing harmful bacteria, inhibiting intestinal FXR, and activating hepatic FXR, ultimately improving lipid metabolism. Arrows indicate promotion (↑) or inhibition (↓).
AMPK – AMP-activated protein kinase, Akt – protein kinase B, Bcl-2 – B-cell lymphoma-2, Bax – Bcl-2-associated X protein, CAT – catalase, FXR – farnesoid X receptor, GSH – glutathione, GSK-3β – glycogen synthase kinase-3β, IL – interleukin, MyD88 – myeloid differentiation primary response 88, NF-κB – nuclear factor kappa B, Nrf2 – nuclear factor erythroid 2-related factor 2, PTP1B – protein tyrosine phosphatase 1B, SOD – superoxide dismutase, SREBP-1c – sterol regulatory element-binding protein-1c, TLR4 – toll-like receptor 4, TNF-α – tumour necrosis factor-alpha.
Antioxidant stress
Under long-term high-sugar and high-fat dietary habits, the liver increases the uptake of excess FFAs present in the circulating blood, which can undergo ectopic deposition, leading to an imbalance between oxidation and antioxidant mechanisms in the liver (55). This results in the accumulation of mitochondrial ROS, causing severe oxidative stress in the liver. Excess ROS can impair mitochondrial function in the liver, leading to significant damage, which exacerbates the deposition of lipid-related molecules in the liver. Nuclear factor E2-related factor 2 (Nrf2) is a key marker reflecting the body's ability to respond to oxidative damage (56). Under normal conditions, Nrf2 binds to the cytoplasmic protein partner molecule Keap1, existing as a complex outside the nucleus in a relatively stable state. When cells encounter harmful external signals, Nrf2 dissociates from Keap1 in the cytoplasm and translocate into the nucleus, promoting the expression of antioxidant-related proteins, including superoxide dismutase (SOD), glutathione peroxidase, and catalase (CAT), thereby increasing the intracellular levels of glutathione (GSH).
AMPK serves as an endogenous central metabolic sensor, regulating cellular energy metabolism, lowering the risk of metabolic-related diseases, and playing a significant role in antioxidant stress (57, 58). Research has shown that activation of the AMPK pathway can promote the expression of Nrf2 and its target genes, thus enhancing the intracellular levels of GSH and improving the body's ability to resist oxidative damage (59). Moreover, in cells experiencing oxidative stress, excess ROS accumulate, which inhibits AMPK activity. GSH can scavenge excess ROS and facilitate the S-glutathionylation of AMPK, further enhancing its activity and alleviating oxidative stress (23, 60). Studies have shown that AS-IV, by activating the AMPK/Nrf2 signaling pathway, increases liver GSH and SOD levels, alleviating the degree of liver oxidative stress and improving the excessive deposition of lipid-related molecules (61, 62). These studies provide evidence that AS-IV can inhibit the excessive accumulation of ROS in cells by activating the AMPK/Nrf2-related molecular pathways, thereby reducing the irreversible damage caused by oxidative stress and enhancing the body's ability to resist oxidative damage, which in turn alleviates the degree of hepatic steatosis.
Anti-inflammatory effects
Long-term high-sugar and high-fat diets can induce hepatic steatosis, triggering compensatory mechanisms to address excessive fat deposition (63). This leads to an increase in mitochondrial fatty acid oxidation. However, the enhanced fatty acid oxidation generates a large amount of ROS, which can promote the gene expression of harmful inflammatory factors such as interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), ultimately leading to liver inflammation. Glycogen synthase kinase-3β (GSK-3β) is known to be associated with the expression of pro-inflammatory factors like IL-6, IL-1β and TNF-α in tissues (64). However, the activity of GSK-3β is inhibited by phosphorylated Akt (p-Akt), which phosphorylates Ser9 of GSK-3β to suppress its activity. Studies have shown that activation of the AMPK signalling pathway increases p-Akt levels, inhibiting GSK-3β activity and subsequently reducing the expression of pro-inflammatory factors such as IL-1β, IL-6 and TNF-α (65). AS-IV can regulate the AMPK/Akt/GSK-3β signalling pathway (23, 66, 67), thereby reducing the expression of harmful pro-inflammatory substances like TNF-α, IL-1β and IL-6 in liver tissues and alleviating liver inflammation (62, 68).
The toll-like receptor-4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear factor kappa B (NF-κB) pathway is a classic signalling pathway mediating inflammation (69). This pathway is significantly activated in liver tissues when NAFLD progresses to NASH. TLR4 is expressed in all liver tissue cells, and in particular, it is the main pathway for Kupffer cells, which originate from the monocyte-macrophage system, to recognise danger signals (70). Under non-inflammatory conditions, NF-κB binds to IκB, forming a stable inactive complex in the cytoplasm (71). When the gut microbiota is disturbed by an unhealthy diet, circulating endotoxins such as lipopolysaccharides and FFAs can activate TLR4 and form a complex with MyD88 (71, 72). This activates the downstream molecule IκB kinase, allowing NF-κB to translocate into the nucleus and promote the expression of downstream inflammatory factors such as TNF-α and IL-6, thereby inducing liver inflammation. Relevant studies have shown that AS-IV can inhibit the gene expression of molecules in the TLR4/MyD88/NF-κB signaling pathway, reducing the levels of harmful inflammatory substances like TNF-α and IL-6 in the blood and alleviating the body's inflammatory response (25). These findings suggest that AS-IV can inhibit the expression of inflammatory factors by regulating the levels of molecules in the AMPK/Akt/GSK-3β and TLR4/MyD88/NF-κB signalling pathways, thereby suppressing the occurrence and progression of inflammation, including in NAFLD.
Anti-apoptosis
NAFLD patients often exhibit mitochondrial dysfunction and endoplasmic reticulum (ER) stress, both of which are major contributors to hepatocyte apoptosis (73). In steatotic hepatocytes, there is an accumulation of ROS that are not cleared in a timely manner. These ROSs inhibit the expression of genes related to the respiratory chain proteins by attacking mitochondrial DNA (mtDNA), leading to impaired respiratory chain function and the subsequent production of more ROS. ROS targets and attacks proteins in the mitochondrial permeability transition pore (MPTP) complex, causing a loss of mitochondrial membrane potential. Subsequently, cytochrome c (Cytc) is released from the mitochondrial intermembrane space into the cytoplasm, where it binds with apoptosis protease activating factor-1 (Apaf-1) and pro-caspase-9 to form an "apoptosome," further activating downstream caspase-3 and triggering apoptosis (73, 74). B-cell lymphoma-2 (Bcl-2) proteins are predominantly located on the outer mitochondrial membrane and the ER membrane (73, 75). These proteins can form heterodimers with the pro-apoptotic protein Bcl-2-associated X protein (Bax), preventing Bax from forming homodimers, thus stabilising mitochondrial membrane permeability and inhibiting the release of Cytc. When ER homeostasis is disrupted, inositol 1,4,5-trisphosphate receptors (InsP3R) release stored Ca2+, leading to intracellular Ca2+ overload, which then activates caspase-12 located on the ER membrane (76, 77). Activated caspase-12 enters the cytoplasm, where it acts on caspase-9, further activating caspase-3 and inducing apoptosis. Research has shown that Bcl-2 can reduce the excessive release of Ca2+ from the ER by inhibiting InsP3R, thereby alleviating ER stress-induced apoptosis (78).
AS-IV has been shown to inhibit hepatocyte apoptosis induced by oxidative stress and inflammatory signalling (79). In a study on NAFLD, AS-IV was found to inhibit the accumulation of lipids induced by palmitic acid (PA) in LO2 cells and to suppress PA-induced oxidative stress and apoptosis in these cells. Furthermore, AS-IV exerts its anti-apoptotic effects by modulating the JNK/p38 (80) and Nrf2/HO-1 (81) signalling pathways. By inhibiting the activation of these pathways, AS-IV reduces cell damage and apoptosis, particularly in response to stressors such as high-fat diets (80, 82). These findings are consistent with other studies, suggesting that AS-IV can reduce apoptosis in various tissues, including brain cells and cardiomyocytes. Additionally, the anti-apoptotic effects of AS-IV are partially mediated by its antioxidant properties, which reduce ROS production, a key initiator of apoptosis (83). By enhancing antioxidant defense, particularly through the Nrf2 pathway, AS-IV helps maintain cellular integrity and decreases the likelihood of apoptosis.
Regulation of gut microbiota dysbiosis and lipid metabolism abnormalities
The human gut harbors a large number of microorganisms, and under physiological conditions, the quantity and ratio of these microbial communities remain relatively stable (84). However, changes in the internal and external environment of the body can lead to gut dysbiosis. The gut and liver are closely connected due to their biological functions and anatomical relationships (85). Most of the liver's blood supply comes from the portal vein, which originates from the gut, linking the gut and liver through the portal circulation. This connection is referred to as the gut-liver axis. Emerging evidence highlights that gut-derived metabolites, such as lipopolysaccharides (LPS) and secondary bile acids, directly modulate hepatic inflammation and lipid metabolism via the portal vein (86). For instance, elevated LPS levels in portal blood activate hepatic Kupffer cells through TLR4 signalling, exacerbating oxidative stress and steatosis in NAFLD (87). Additionally, dysbiosis-induced alterations in bile acid metabolism impair FXR signalling, further disrupting hepatic lipid homeostasis (88). These mechanisms underscore the gut-liver axis as a pivotal therapeutic target for NAFLD.
This intricate crosstalk implies that liver metabolic dysfunction can reciprocally exacerbate gut dysbiosis. For example, impaired hepatic bile acid synthesis disrupts intestinal barrier integrity, allowing translocation of bacterial products like endotoxins into the portal circulation (89, 90). Xue et al. (91) found that faecal microbiota transplantation can effectively improve the treatment outcomes for NAFLD patients. Kaden-Volynets et al. (92) found that, compared to normal mice, germ-free mice fed a high-fat diet showed increased body weight and lipid metabolism abnormalities, although their livers remained normal without any signs of steatosis. Fu et al. (93) discovered that changes in the firmicutes/bacteroidetes ratio can alter blood lipid levels. This mechanism may be related to bile acids secreted by the gut microbiota, which promote fat absorption and activate G protein-coupled bile acid receptor 1 and other bile acid receptors, thus affecting lipid metabolism.
Several studies have shown that AS-IV can restore gut microbiota dysbiosis (94, 95). For example, AS-IV treatment has been shown to increase the abundance of beneficial bacteria such as Akkermansia muciniphila, lactobacilli and bifidobacteria strains, while reducing harmful bacteria such as Escherichia coli and Streptococcus strains capable of causing different pathologies. These changes in the gut microbiota composition are associated with improvements in metabolic and inflammatory markers in NAFLD models (96). Specifically, AS-IV has been shown to increase the production of short-chain fatty acids (SCFAs) and regulate macrophage polarization, both of which play key roles in maintaining gut barrier integrity and modulating inflammation (21, 97). Furthermore, studies indicate that the effects of AS-IV on the gut microbiota may be mediated through the regulation of the NLRP3 inflammasome. By reshaping the gut microbiota and enhancing the production of bacteria such as Butyricicoccus, AS-IV not only alleviates the inflammatory burden but also contributes to the restoration of normal liver function (21). Zhai's study indicated that AS-IV alleviates diet-induced hepatic steatosis by regulating gut microbiota and bile acid metabolism (24). It reduces bile salt hydrolase (BSH) activity, increases taurine-β-muricholic acid levels, and inhibits intestinal farnesoid X receptor (FXR). This results in the activation of hepatic FXR, increased glucagon-like peptide-1 (GLP-1), decreased ceramide, and inhibition of SREBP-1c, ultimately reducing liver fat accumulation (24).
In conclusion, AS-IV exerts its therapeutic effects on NAFLD through multiple mechanisms, including improving insulin resistance, inhibiting oxidative stress, reducing inflammation, suppressing hepatocyte apoptosis, and regulating gut microbiota and bile acid metabolism. These actions, mediated through the regulation of key molecular pathways such as AMPK, Nrf2 and SREBP-1c, significantly improve lipid metabolism abnormalities and reduce hepatic steatosis, providing new potential strategies for the treatment of NAFLD (Table II).
Table II. Pharmacological effects and mechanisms of astragaloside IV in non-alcoholic fatty liver disease
Akt – protein kinase B; AMPK – AMP-activated protein kinase, Bax – Bcl-2-associated X protein, Bcl-2 – B-cell lymphoma-2, Cytc – cytochrome c, FXR – farnesoid X receptor, GLP-1 – glucagon-like peptide-1, GSH – glutathione, GSK-3β – glycogen synthase kinase-3β, IL-6 – interleukin-6, NAFLD – non-alcoholic fatty liver disease, NF-κB – nuclear factor kappa B, NLRP3 – NLR family pyrin domain containing 3, Nrf2 – nuclear factor erythroid 2-related factor 2, PTP1B – protein tyrosine phosphatase 1B, ROS – reactive oxygen species, SOD – superoxide dismutase, SREBP-1c – sterol regulatory element-binding protein 1c, TLR4 – toll-like receptor 4, TNF-α – tumour necrosis factor-alpha.
CURRENT CHALLENGES AND FUTURE PERSPECTIVES
Currently, the mainstream treatments for NAFLD mainly include lifestyle interventions, pharmacological treatments and surgical procedures (97). Among them, lifestyle interventions that regulate basic dietary and exercise habits are widely promoted. However, achieving significant improvement in liver function requires at least a 5 % body weight reduction, while reversing liver fibrosis requires a weight loss of more than 10 %, which must be sustained for at least one year. Most patients find it difficult to maintain these changes. Surgical treatments primarily include bariatric surgery and liver transplantation; however, there is insufficient evidence to support the use of bariatric surgery for the treatment of NAFLD, and the recurrence rate of NAFLD after liver transplantation is as high as 50 %, with a higher risk of cardiovascular complications. Therefore, pharmacological treatments are highly anticipated.
Faced with the growing clinical demand, the pharmacological market for NAFLD still faces a significant gap (98, 99). Multiple NAFLD drugs are under development both domestically and internationally, but to date, only resmetirom (THRβ agonist), developed by Madrigal Pharmaceuticals (USA), has recently been approved by the U.S. Food and Drug Administration (FDA) for the treatment of NASH with liver fibrosis. Other promising drugs in development include lanifibranor (a pan-PPAR agonist) in phase III clinical trials (100), and semaglutide (a GLP-1 receptor agonist) (101). In addition to being used as monotherapy, a phase IIa clinical trial of a combination therapy (semaglutide + cilofexor + firsocostat) has also reached the primary endpoint and demonstrated good safety (102). Furthermore, drugs such as BIO89-100, efruxifermin. and VK2809, have also shown potential in clinical trials for NAFLD and NASH, although they are still at different clinical stages and require further research to confirm their efficacy and long-term safety (103–105). At the same time, compared to monotherapy, combination therapies have shown statistically significant improvements in liver fat accumulation and liver injury. As with most disease treatments, the treatment of NAFLD/NASH requires weighing the benefits against potential side effects (106). For example, resmetirom, while treating NASH and liver fibrosis, still carries a certain risk of liver toxicity, cholelithiasis and cholecystitis, and not all patients benefit from the drug, as 26 % of patients show no response. Additionally, in the early stages of the NAFLD disease spectrum, there are no obvious clinical manifestations, and NASH is often not detected promptly. This leads to some patients not seeking pharmacological treatments in a timely manner. Therefore, there remains a need to develop new drugs for NAFLD and explore new therapeutic targets (Table III).
Table III. Comparative data on astragaloside IV and other treatments against non-alcoholic fatty liver disease (NAFLD)
Akt – protein kinase B, ALT – alanine aminotransferase (also known as serum glutamate-pyruvate transaminase, SGPT)/AST – aspartate aminotransferase (also known as serum glutamic oxaloacetic transaminase, SGOT), AMPK – AMP-activated protein kinase, AS-IV – astragaloside IV, FGF21-Fc – fibroblast growth factor 21, Fc fusion variant, GLP-1 – glucagon-like peptide-1, LDL-C – low-density lipoprotein cholesterol, NAFLD – non-alcoholic fatty liver disease, NAFL – non-alcoholic fatty liver, NASH – non-alcoholic steatohepatitis, Nrf2 – nuclear factor erythroid 2-related factor 2, PPAR – peroxisome proliferator-activated receptor, Pro-C3 – plasma Pro-C3 (N-terminal type III collagen propeptide), THR-β – thyroid hormone receptor beta, TLR4 – toll-like receptor 4, N/A – not applicable.
AS-IV, the major bioactive component of Astragalus membranaceus, is recognised as a quality control marker for this herb in traditional medicine (107). Preclinical studies demonstrate that AS-IV reduces hepatic steatosis and inflammation in NAFLD models by targeting multiple pathological pathways (23, 24, 62). Specifically, it activates AMPK/Nrf2 signalling to mitigate oxidative stress and lipid accumulation, while suppressing TLR4/NF-κB-mediated inflammatory responses. Additionally, AS-IV modulates gut microbiota composition and bile acid metabolism, further contributing to its therapeutic efficacy (24, 94). These multi-target effects position AS-IV as a promising candidate for NAFLD treatment, particularly in addressing metabolic dysregulation and inflammation-driven liver injury.
Although AS-IV has demonstrated broad potential in the treatment of NAFLD, there are still some limitations in current research. First, most studies remain at the cellular and animal model stages, lacking clinical data to support their efficacy. While animal studies have shown that AS-IV effectively alleviates liver steatosis and improves metabolic disorders, its efficacy, safety and bioavailability in different populations still require further validation. Additionally, the specific mechanisms of AS-IV, particularly how it coordinates between different molecular pathways, remain unclear. Future research should focus on clinical trials of AS-IV to confirm its therapeutic effects in NAFLD patients, explore the optimal dosage, treatment regimens, and assess the long-term safety of its use. Furthermore, as a component of TCM, the combined use of AS-IV with other drugs has not been fully explored. For example, the combination of AS-IV with other drugs, such as insulin sensitisers or anti-inflammatory agents, may have synergistic effects, justifying the need for further investigation. Additionally, the pharmacokinetic properties of AS-IV and its metabolic processes in the liver need additional studies to enhance its therapeutic effects and reduce side effects.
CONCLUSIONS
AS-IV, a naturally derived bioactive compound, has demonstrated significant therapeutic potential in the treatment of NAFLD. It improves the pathological state of NAFLD through multiple mechanisms, including enhancing insulin resistance, regulating lipid metabolism, inhibiting oxidative stress, exerting anti-inflammatory and anti-apoptotic effects, and modulating gut microbiota and bile acid metabolism. Its actions, mediated through the regulation of key molecular pathways such as AMPK, Nrf2 and SREBP-1c, reduce hepatic steatosis and related inflammation, offering a new strategy for the treatment of NAFLD. However, despite the promising results in animal models, there is still a lack of extensive clinical data to validate its efficacy and safety in diverse populations. Future research should focus on clinical trials, pharmacokinetic analysis, and the combined application of AS-IV with other drugs to further elucidate its mechanisms in treating NAFLD and enhance its clinical applicability.
List of acronyms, abbreviations, symbols. – Akt – protein kinase B, ALT – alanine aminotransferase (also known as serum glutamate-pyruvate transaminase, SGPT)/AST – aspartate aminotransferase (also known as serum glutamic oxaloacetic transaminase, SGOT), AMPK – AMP-activated protein kinase, AS-IV – astragaloside IV, Bax – Bcl-2-associated X protein, Bcl-2 – B-cell lymphoma-2, BSH – bile salt hydrolase, CAT – catalase, Cytc – cytochrome c, ECM – extracellular matrix, ER – endoplasmic reticulum, FasL – Fas ligand, FFA – free fatty acid, FGF21-Fc – fibroblast growth factor 21-Fc fusion variant, FXR – farnesoid X receptor, GLP-1 – glucagon-like peptide-1, GSH – glutathione, GSK-3β – glycogen synthase kinase-3β, HCC – hepatocellular carcinoma, HSC – hepatic stellate cell, IL-1β – interleukin-1β, IL-6 – interleukin-6, IR – insulin resistance, Keap1 – Kelch-like ECH-associated protein 1, LDL-C – low-density lipoprotein cholesterol, MPTP – mitochondrial permeability transition pore, MyD88 – myeloid differentiation factor 88, NAFLD – non-alcoholic fatty liver disease, NAFL – non-alcoholic fatty liver, NASH – non-alcoholic steatohepatitis, NF-κB – nuclear factor kappa B, NLRP3 – NLR family pyrin domain containing 3, Nrf2 – nuclear factor erythroid 2-related factor 2, PA – palmitic acid, PPAR – peroxisome proliferator-activated receptor, Pro-C3 – plasma Pro-C3 (N-terminal type III collagen propeptide), PTP1B – protein tyrosine phosphatase 1B, ROS – reactive oxygen species, SCFA – short-chain fatty acid, SOD – superoxide dismutase, SREBP-1c – sterol regulatory element-binding protein 1c, TG – triglyceride, THR-β – thyroid hormone receptor beta, TLR4 – toll-like receptor 4, TNF-α – tumour necrosis factor-alpha, VLCFA – very long-chain fatty acid.
Funding. – This work was supported by the Pudong New Area Traditional Chinese Medicine Inheritance and Innovation Development Pilot Project (YC-2023-0610), Traditional Chinese Medicine research project of Shanghai Health Commission (2024QN092), and Scientific Research Program of Shanghai Pudong New Area Health Commission (the General Program) (PW2022A-90).
Conflict of interest. – The authors declare no conflict of interest.
Authors contributions. – Conceptualisation, H.W. and S.W.; writing, original draft preparation, S.W. and Y.J.; writing, review and editing, C.L., L.T., T.G., and S.S.; supervision, S.S. and T.G.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
