1. Introduction
Vitamins are essential micronutrients required for the normal functioning of living organisms. There are water-soluble and fat-soluble vitamins. Vitamin E belongs to the group of fat-soluble vitamins. It consists of eight compounds: four tocopherols and four tocotrienols. Each of these groups possesses four isoforms α, β, γ and δ based on the location of a specific methyl group on chromanol at positions 5, 7 and 8 (Schneider, 2005).
The most biologically active form of vitamin E is α -TOH, which is preferentially absorbed and retained in the body since the liver differentiates the forms of vitamin E and excretes α -TOH into the plasma. Vitamin E is an antioxidant that plays an important role in cellular respiration. Vitamin E is present in foods that contain fat and can be stored in the fatty tissues of animals and humans. Since it is fat-soluble, vitamin E does not need to be consumed every day. Among foods, the best source of vitamin E is vegetable oil. It is also found in various other foods such as nuts and seeds that contain high amounts of α -TOH, and significant amounts are also available in green leafy vegetables and fortified cereals (Arnarson, 2023).
Vitamin E, especially α -TOH, was studied because of its anti-bacterial, anti-inflammatory, and anti-cancer activity (Gupta et al., 2016; Gamna et al., 2021). So far, α -TOH has been detected and quantified with different techniques: spectroscopic techniques (Raman (RAMAN) spectroscopy, fourier-transformed infrared spectroscopy (FTIR), ultraviolet-visible (UV/Vis) spectrophotometry), high-performance liquid chromatography (HPLC) and spectrophotometric methods (2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu etc.) (Lee et al., 2004; Sawicki et al., 2020; Lampen et al., 2003; Orsavová et al, 2019; Marcos et al., 2014; Korchazhkina et al, 2006; Webster, 2022). Normal phase and reversed-phase HPLC were used to determine α -TOH in olive oil (Bakre et al., 2015), and FTIR- attenuated total reflectance (ATR) was also used to detect and quantify α -TOH in vegetable oils (Silva et al., 2009). Oxidation products of vitamin E hydrolysis and oxidation were also monitored using spectroscopic methods (UV-Vis, Raman, FTIR, electron paramagnetic resonance (EPR)) and voltammetry, and they were combined with fast photoinitiated excitation and time-resolved spectroscopy for the detection of short-lived species (Webster, 2022).
Until now, the electrochemical properties of α -TOH have not been extensively studied, so the main goal of our research was to characterize α -TOH using cyclic voltammetry (CV) and differential pulse voltammetry (DPV), and to detect α -TOH with HPLC in real samples (parsley, kale, and a powdered dietary supplement).
2. Materials and methods
Electrochemical measurements
All commercially available chemicals were of reagent grade and were used as purchased. Lithium chloride (LiCl) was purchased from VWR chemicals, ethanol absolute, p.a. was purchased from Gram-mol and α-tocopherol (purity 99,9%) was purchased from Sigma Aldrich). CV and DPV were used to test the electrochemical properties and oxidation mechanism of α -TOH. Electrochemical measurements were performed at room temperature ( θ= 25 ± 1 °C) in a three-electrode voltammetric cell connected to a PalmSens potentiostat/galvanostat (PalmSens BV, Utrecht, The Netherlands) equipped with PSTrace 4.2 software. The working electrode was a glassy carbon electrode area, A = 0.07 cm2) and it was polished before each measurement with α−Al2O3 (0.05 µm, Als, Japan). Ag/AgCl electrode for non-aqueous media was used as a reference electrode, while a platinum wire was used as the counter electrode. Electrochemical measurements were performed in a 15 mL cell and before each experiment, the system was purged with Ar5 ( φAr = 99.999%). Stock solution of α-tocopherol ( c = 1 mol/dm3) was prepared in Milli Q-Water (MQ H2O) and then the appropriate volume of stock solution was diluted with the appropriate solvent (mixture of ethanol and MQ H2O in 30:70 volume ratio) to the desired concentration before each measurement. Cyclic voltammograms were recorded in a potential range from -0.4 V to 1.0 V in a blank solution and a solution of α -TOH and the scan rate varied from 50 mV/s to 300 mV/s. Parameters used for differential pulse voltammetry were: scan rate of 5 mV/s, scan increment of 5 mV, pulse width of 70 ms, and pulse amplitude of 25 mV. All the potentials in this work are referred to as Ag/AgCl reference electrodes.
HPLC analysis
Samples
Dry parsley, dry kale, and a powdered dietary supplement were used as real samples. Parsley was bought dried, kale was fresh and then dried for 24 hours at 50 °C. Parsley and kale were ground in a home use mill and then sieved. A powdered dietary supplement was used without previous treatments. All samples were purchased from a local store. Real samples were stable for several days, so that the samples could be prepared a few days prior to analysis.
Calibration curve
The calibration curve was constructed in a range of concentrations from 5 to 60 mg/dm3. All standard solutions were prepared by diluting a 100 mg/dm3 stock solution with methanol. Stock solution was prepared from standard α -TOH (purity 99,9% Sigma Aldrich). All prepared standard solutions were kept at 4˚C.
For the measurement of α -TOH concentration, HPLC system Nexera X2 (Shimadzu) with a UV/Vis detector was used coupled with a Shim-pack GIST C-18 (150 mm x 4.6 mm, 5 µm) (Shimadzu) column, used for the separation. The mobile phase consisted of methanol (J. T. Baker, Poland) and isopropanol (Fisher Chemical, UK) (methanol: isopropanol = 45:55, V/V), flow rate - 0.7 mL/min, sample injection volume was 20 μL, and total analysis time for one sample was 10 min. All measurements were performed at 295 nm and at room temperature.
Extraction procedure
The extraction of α -TOH was carried out using methanol (Chen et al., 2005). The extraction mixture consisted of methanol and a 0.2% methanolic solution of BHT (2,6-Di-tert-butyl-4-methylphenol) (Acros Organics, Germany).
Analysis of real samples
Real samples were prepared by adding ground, sieved, and then weighed samples to amber tubes along with methanol (MetOH) and BHT. Sample: extraction mixture ratio for parsley and kale was 1:50 and for a dietary powdered supplement 1:1.25. The samples were vortexed for 1 minute and left in the dark for 1 hour. After 1 hour, the samples were centrifuged at 6000 rpm for 10 minutes and filtered with a syringe filter (pore size - 0.20 µm). The prepared samples were analyzed by means of HPLC with a previously used method (Martino et al., 2014).
Stability of real samples
The stability of parsley and kale was tested on three different samples with an extraction mixture ratio: of 1:30, 1:40, 1:50 for parsley, and 1:50, 1:80 and 1:100 for kale. Concentration of α-tocopherol was measured after 1 hour and after 96 hours for parsley and after 72 hours for kale. During waiting times of 96 hours and 72 hours, the samples were kept at 4 °C in the dark.
3. Results and discussion
CV and DPV
In order to find the optimal medium to study the electrochemical properties of α -TOH, cyclic (Fig. 1a) and differential pulse (Fig. 1b) voltammograms were recorded in pure ethanol and in mixtures of ethanol and water in a 50:50, 70:30 and 80:20 volume ratio. Both methods have shown that the most pronounced anodic peak current of α -TOH was present in an ethanol-aqueous mixture of 70:30 volume ratio, so all the experiments were performed in this medium.
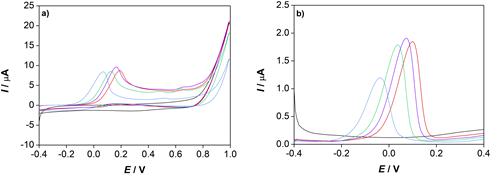
Figure 1. a) Cyclic voltammograms and b) differential pulse voltammograms of (▬) a blank solution and α -TOH ( c = 5x10-4 mol/dm3, Ic = 0.01 mol/dm3 LiCl) recorded in: (▬) ethanol and a mixture of ethanol and water in a (▬) 50:50, (▬) 70:30 and (▬) 80:20 volume ratio.
In the cyclic voltammogram recorded in a mixture of ethanol and water in a 70:30 volume ratio (Fig. 2), one oxidation peak of α -TOH was detected at the potential Ep, a = 0.175 V, which corresponds to the irreversible oxidation of α -TOH. In the oxidation of α -TOH, one electron is exchanged and the cation radical ( α -TOH+·) is formed. The cation radical rapidly deprotonates into the neutral phenoxyl radical ( α -TO·), which further oxidizes at the electrode surface to form a diamagnetic cation ( α -TO+) (Webster, 2022).
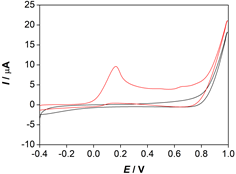
Figure 2. Cyclic voltammograms of: (▬) α -TOH ( c = 5x10-4 mol/dm3, Ic = 0.01 mol/dm3 LiCl) and (▬) a blank solution-mixture of ethanol and water in a 70:30 volume ratio.
In Fig. 3a, cyclic voltammograms of α -TOH recorded on the glassy carbon electrode at different scan rates ( ν = 50-300 mV/s) are shown. It can be seen that the oxidation peak current and the oxidation peak potential increase with the increase of a scan rate. The logarithm of anodic peak current ( Ip, a) is a linear function of the logarithm of a scan rate (log ν ) (Fig. 3b) with a slope around 0.5, which indicates that the oxidation of α -TOH is a diffusion-controlled process (David et al., 2018).
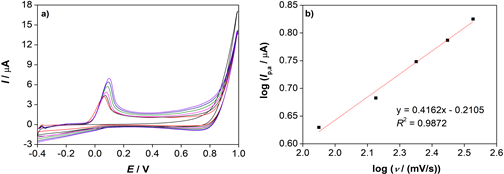
Figure 3. a) Cyclic voltammograms of (▬) a blank solution and α -TOH ( c = 5x10-4 mol/dm3, Ic = 0.01 mol/dm3 LiCl) at the scan rate, ν = (▬) 50, (▬) 100, (▬) 150, (▬) 200, (▬) 250 and (▬) 300 mV/s. b) the logarithm of oxidation peak current (log Ip, a) as a function of the logarithm of scan rate (log ν ).
DPV was also used to study the electrochemical properties of α -TOH. In a differential pulse voltammogram (Fig. 4a), one anodic peak current was detected at the potential, Ep, a = 0.070 V, which also corresponds to the oxidation of α -TOH (Shu-Guo et al., 2008). The oxidation peak current ( Ip, a) increases with the increase of α -TOH mass concentration (Fig. 4b). Linear correlation of Ip, a vs α -TOH mass concentration ( γ) was obtained with the equation: Ip, a (μA) = 0.0126 γ / (mg/dm3) + 0.0358 ( R2= 0.9912) in the concentration range from 0.43 mg/dm3 to 70.41 mg/dm3.
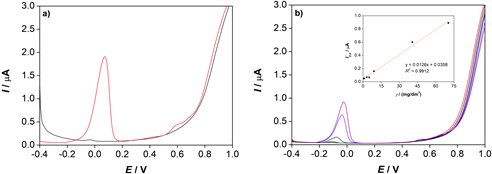
Figure 4. a) Differential pulse voltammograms of: (▬) α -TOH( c = 5x10-4 mol/dm3, Ic = 0.01 mol/dm3 LiCl) and (▬) a blank solution and b) differential pulse voltammograms of α -TOH at different mass concentrations, γ = (▬) 0.43, (▬ ) 1.10, (▬) 3.09, (▬) 5.07, (▬) 9.02, (▬) 40.69 and (▬) 70.41 mg/dm3. Inset: Anodic peak current ( Ip,a) as a function of mass concentration ( γ) of α -TOH.
HPLC
Calibration curve
Chromatograms obtained by measuring series of standard solutions of α -TOH are shown in Fig. 5a. The calibration curve constructed using the Lab Solutions software is shown in Fig. 5b. The value of the coefficient of determination was R2 = 0.9992 and we can conclude that the measured values and the regression line correlate.
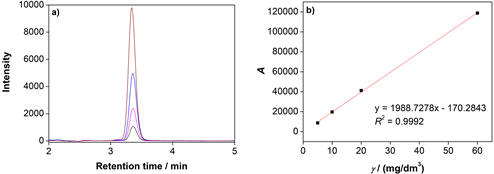
Figure 5. a) Comparison of chromatograms obtained by measuring the absorbance of a series of standard α -TOH solutions (▬ 5 mg/dm3, ▬ 10 mg/dm3, ▬ 20 mg/dm3, ▬ 60 mg/dm3) and b) calibration curve of α -TOH.
Analysis of real samples
Results of the HPLC analysis of real samples are shown in Table 1. Parsley had the highest concentration of α -TOH 8.267 mg/kg, while kale had a significantly lower concentration of 1.492 mg/kg. The lowest concentration was measured in a powdered dietary supplement which amounted to 0.067 mg/kg. The determined concentration of α -TOH in the powdered dietary supplement was not in accordance with the supplement declaration. The resulting discrepancy could be explained by the complex composition of the supplement, which contains additional substances that interfere with the extraction and analysis of the sample itself.
Table 1. Concentration of α -TOH in real samples obtained by HPLC analysis.
Stability of real samples
The stability of parsley and kale samples is shown in Tables 2 and 3. As can be seen from the Tables 2 and 3, the decrease in the concentration of α -TOH in parsley was not higher than 1.72% after 96 hours, and the concentration of α -TOH in kale after 72 hours increased by 4% in the sample with an extraction mixture ratio of 1:50, and for the sample with an extraction mixture ratio of 1:80, the concentration decreased by 6.6%, but for the sample with a solvent ratio of 1:100, the concentration of α -TOH did not change.
Table 2. Results of the stability test for parsley for different samples: extraction mixture ratio and two extraction times.
| Extraction time | 1 h | 96 h | |
|---|---|---|---|
| Sample: extraction mixture ratio | γ (α -TOH ) / mg/kg | ||
| parsley | 1:30 | 6.939 | 6.848 |
| parsley | 1:40 | 6.960 | 6.840 |
| parsley | 1:50 | 6.950 | 6.850 |
Table 3. Results of the stability test for kale for different samples: extraction mixture ratio and two extraction times.
4. Conclusion
In this study, CV and DPV were used for the electrochemical characterization of α -TOH in an ethanol-aqueous mixture in a 70:30 volume ratio. Cyclic and differential pulse voltammograms have shown one oxidation peak which corresponds to the oxidation of α -TOH. In the oxidation of α -TOH, rapid two-electron oxidation, coupled with one proton loss (–2e−/–1H+) occurred, and a diamagnetic cation ( α -TO+) was formed. Oxidation of α -TOH is an irreversible and a diffusion-controlled process since the linear correlation between anodic peak current, Ip, a, and the square root of scan rate ( ν )1/2 was observed. It was also determined that the anodic peak current of α -TOH increases with the increase of its concentration (linear response was obtained in the concentration range from 0.43 mg/dm3 to 70.41 mg/dm3). The obtained values of α -TOH in real samples with HPLC were 8.267 mg/kg for parsley, 1.492 mg/kg for kale, and 0.067 mg/kg for powdered dietary supplement.
Author Contributions: D. G.: formal analysis, writing-original manuscript draft preparation, review, and editing. V. D. performed the HPLC and voltammetric measurements, data collection, formal analysis. O. G.: conceptualized the project, analyzed the experimental data, writing - original manuscript draft preparation. M. M.-K.: project conceptualization, supervision, review, and editing. All authors read and approved the final manuscript.
Funding: This research was funded by the European Structural and Investment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care, Josip Juraj Strossmayer University of Osijek (grant #KK.01.1.1.01.0010).
Conflicts of Interest: The authors declare no conflict of interest.
