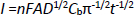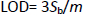Introduction
Cancer is a class of diseases characterized by out-of-control cell growth. Due to ineffective drugs, cancer is the cause of most deaths worldwide. Therefore it's essential to develop cancer research in order to identify causes and develop strategies for prevention, diagnosis, treatments and cure [1,2].
5-Fluorouracil is an antimetabolite fluoropyrimidine analog prescribed as a chemotherapy drug. 5-fluorouracil, a derivative of uracil in which the 5th positioned hydrogen is replaced by fluorine, is used as an excellent antineoplastic agent in treatment of cancer such as colorectal, breast, stomach, pancreatic, and cervical. It acts on cancer cells by directly incorporating into nucleic acids and inhibiting the thymidylate synthase enzyme, which is involved in nucleotide synthesis [3-6].
However, an overdose of 5-Fluorouracil may result in very toxic side effects such as mucositis, leukopenia, nausea, diarrhea, alopecia, neurotoxicity, ocular toxicity, and cardiac toxicity by accumulation. Consequently, controlling the dose of this drug in biological samples and studying the purity of its pharmaceutical forms can help manage its side effects [7-9].
Currently, there are several analytical techniques for the determination of 5-fluorouracil, such as capillary electrophoresis [10], high-performance liquid chromatography [11], Raman spectroscopy [12] and mass spectrometry [13]. In spite of their good performance, these techniques are generally expensive, time-consuming and require relatively complex procedures for sample preparation.
The use of electrochemical sensors presents an alternative to the aforementioned analyses for the determination of 5-Fluorouracil. Electrochemical methods are highlighted and deserve special attention since they present high sensitivity, selectivity, reproducibility, low cost, and rapid response generation, and related to dyes, they present functional groups that can undergo redox reactions, enabling their determination. Among the electrochemical techniques, voltammetry deserves special attention once different sensors (modified or not) can be applied [14-27].
Magnification of the above properties can be achieved by the application of chemically modified electrode. Electrode modification in analytical chemistry has always been an interesting field. Modifiers effectively transport electrons between the electrodes to an analyte. It works by minimizing the over-potential required for the electrode reactions and increasing the electrode's sensitivity and selectivity [28-39].
With the advent of nanoscience, various kinds of nanoparticles are utilized for electrochemical sensors in several analytical methods due to their unique character. Owing to their small size (normally in the range of 1–100 nm), nanoparticles exhibit unique chemical, physical and electronic properties that are different from those of respective bulk materials and can be used to construct novel and improved sensing devices. Compared with the traditional macroelectrodes, nanostructured electrodes show an increased mass-transport rate, a decreased influence of the solution resistance and a higher signal-to-noise ratio [40-54].
In order to develop a miniaturized sensor, screen-printed electrodes (SPEs) are a valuable choice. SPEs produced by printing different inks on plastic or ceramic supports are gaining widespread applicability for the electrochemical monitoring field. Recently, SPEs have emerged as a simple, disposable, nontoxic and low-cost alternative to mercury-based and conventional solid electrodes for the voltammetric determination of many substances. Moreover, they have been shown to be a convenient substrate for nanoparticle modification [55-58].
In the present work, we synthesized CuFe2O4 nanoparticles (CuFe2O4 NPs) and screen-printed graphite electrodes were modified with CuFe2O4 NPs using the drop-casting method. The resulting modified electrode is applied to the determination of 5-fluorouracil by differential pulse voltammetry.
Experimental
Apparatus and chemicals
All the electrochemical measurements were carried out on a PGSTAT302N potentiostat/galvanostat Autolab. The measurement cell consisted of SPGE (DropSens; DRP-110: Spain) containing a graphite counter electrode, a graphite working electrode, and a silver pseudo-reference electrode. Solution pH values were determined using a 713 pH meter combined with a glass electrode (Metrohm, Switzerland). 5-fluorouracil and all chemicals used were of analytical grade and were used as received without any further purification and were obtained from Merck and Sigma Aldrich. Orthophosphoric acid was utilized to prepare the phosphate buffer solutions (PBSs), and sodium hydroxide was responsible for adjusting the desired pH values (pH range between 2 and 9).
Preparation of modified electrode
CuFe2O4 NPs/SPGEs were prepared by modifying the bare working electrode of an SPGE using the drop-casting method. Briefly, 4 μL of the solution of CuFe2O4 NPs (1 mg/mL) were dropped onto the working electrode surface and dried at room temperature. The obtained electrode was noted as CuFe2O4 NPs/SPGE.
The surface areas of the CuFe2O4 NPs/SPGEs and the un-modified SPGEwere obtained by CV using 1 mM K3Fe(CN)6 at various scan rates. Using the Randles–Sevcik equation for CuFe2O4 NPs/SPGEs, the electrode surface was found to be 0.113 cm2 which was about 3.6 times greater than un-modified SPE.
Results and discussion
Electrochemical behavior of 5-fluorouracil at the surface of various electrodes
The effect of the electrolyte pH on the oxidation of 50.0 μM 5-fluorouracil was investigated at CuFe2O4 NPs/SPGE using differential pulse voltammetry (DPV) measurements in the PBS in the pH range from 2.0 to 9.0. According to the results, the oxidation peak current of 5-fluorouracil depends on the pH value and increases with increasing pH until it reaches the maximum at pH 7.0 and then decreases at higher pH values. The optimized pH corresponding to the higher peak current was 7.0, indicating that protons are involved in the reaction of 5-fluorouracil oxidation.
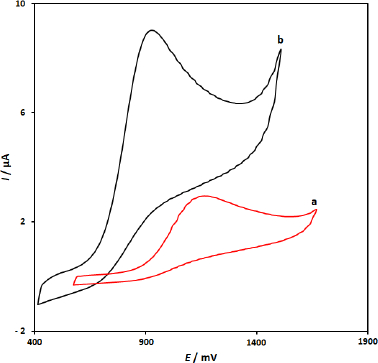
Figure 1 displays CV responses from the electrochemical oxidation of 100.0 μM 5-fluorouracil at the surface of CuFe2O4 NPs/SPGE (curve b) and bare SPGE (curve a). The results showed that the oxidation of 5-fluorouracil is very weak on the surface of the bare SPGE, but the presence of CuFe2O4 NPs in SPGE could enhance the peak current and decrease the oxidation potential (decreasing the overpotential). A substantial negative shift of the currents starting from oxidation potential for 5-fluorouracil and a dramatic increase of the current indicates the catalytic ability of CuFe2O4 NPs/SPGE to 5-fluorouracil oxidation. The results showed that the using of CuFe2O4 nanoparticles (curve b) definitely improved the characteristics of 5-fluorouracil oxidation, which was partly due to excellent characteristics of CuFe2O4 NPs, such as good electrical conductivity and high chemical stability.
Effect of scan rate on the determination of 5-fluorouracil at CuFe2O4 NPs/SPGE
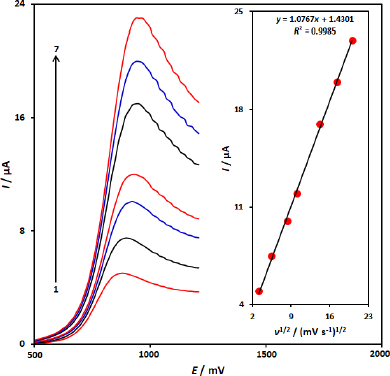
The influence of potential scan rate (ν) on Ip of 100.0 μM 5-fluorouracil at the CuFe2O4 NPs/SPGE was studied by linear sweep voltammetry (LSV) at various sweep rates (Figure 2). As shown inFigure 2, the peak currents of 5-fluorouracil grow with the increasing scan rates and there are good linear relationships between the peak currents and ν1/2 (square root of scan rate) (Figure 2 inset). The regression equation is Ipa = 1.0767 ν1/2 +1.4301 (Ipa/ μA, ν / mV s-1, R2= 0.9985), indicating the oxidation process of 100.0 μM 5-fluorouracil at the CuFe2O4 NPs/SPGE was diffusion-controlled.
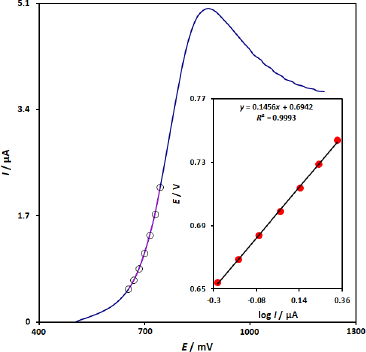
To obtain further information on the rate-determining step, the Tafel plot for oxidation of 100.0 μM 5-fluorouracil at the surface of CuFe2O4 NPs/SPGE using the data derived from the raising part of the current–voltage curve has been recorded inFigure 3. Using the slope of the Tafel plot at a scan rate of 10 mV s-1, the value of electron transfers coefficient (α) was determined as 0.6, confirming an irreversible process for the oxidation of 5-fluorouracil on the surface of CuFe2O4 NPs/SPGE.
Chronoamperometric studies
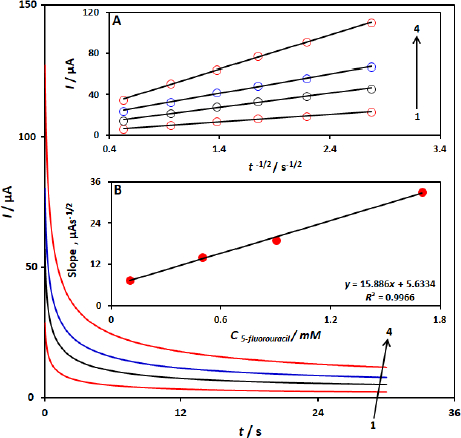
The electrochemical oxidation of 5-fluorouracil by a CuFe2O4 NPs/SPGE was also studied by chronoamperometry. Chronoamperometric measurements of different concentrations of 5-fluorouracil at CuFe2O4 NPs/SPGE were done by setting the working electrode potential at 1000 mV (Figure 4). In chronoamperometric studies, we have determined the diffusion coefficient, D, of 5-fluorouracil. The experimental plots of I versus t-1/2 with the best fits for different concentrations of 5-fluorouracil were employed (Figure 4 A). The slopes of the resulting straight lines were then plotted versus the 5-fluorouracil -concentrations (Figure 4 B), from whose slope and using the Cottrellequation (1):
We calculated a diffusion coefficient of 8.3×10-5 cm2 s-1 for 5-fluorouracil.
Calibration curve and limit of detection
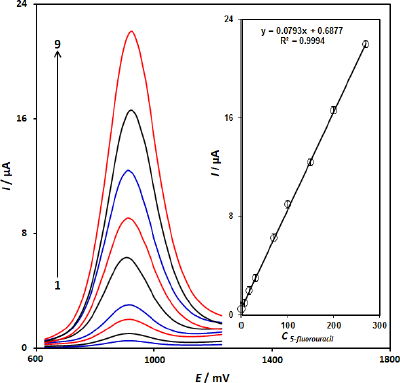
Since DPV has a much higher current sensitivity than cyclic voltammetry, we used the DPV method for the determination of 5-fluorouracil (Step potential=0.01 V and pulse amplitude=0.025 V).Figure 5 shows DPVs of different concentrations of 5-fluorouracil and the obtained calibration curve. The results showed a linear segment for 5-fluorouracil concentration from 0.1 to 270.0 μM 5-fluorouracil (Figure 5), with a regression equation of Ip = 0.0793C5-fluorouracil + 0.6877 (R2= 0.9994, n=9). The detection limit, LOD, was obtained by using theequation (2):
where Sb is the standard deviation of the blank response (n=15) and m is the slope of the calibration plot. The limit of detection was determined to be 0.03 μM for 5-fluorouracil.
Real sample analysis
To investigate the applicability of the proposed sensor for the voltammetric determination of 5-fluorouracil in real samples, we selected urine and 5-fluorouracil injection samples for the analysis of 5-fluorouracil contents. The 5-fluorouracil contents were measured after sample preparation using the standard addition method. The results are given inTable 1. According to the table, the recovery values within 98.0-103.6 % confirm the powerful ability of CuFe2O4 NPs/SPGE for the determination of 5-fluorouracil in real samples.
Conclusions
The fabrication of sensors for the measurement of 5-fluorouracil was achieved using screen-printed graphite electrodes modified with CuFe2O4 NPs. The CuFe2O4 NPs remarkably decreased overvoltage and improved the electrochemical response of 5-fluorouracil in terms of specificity, sensitivity and current response. Under optimized conditions, differential pulse voltammetry exhibited linear dynamic ranges from 0.1-270.0 μM with a detection limit of 0.03 μM. Also the CuFe2O4 NPs/SPGE was used to detect 5-fluorouracil in real samples and produced satisfactory results.