1. Introduction
A chronic inflammatory state presents a main reason for the development of different chronic pathologies such as cancers, infections, obesity, cardiovascular and neurodegenerative diseases. The results of epidemiological studies showed the connection between a diet rich in fruits and vegetables with reduced incidence of inflammatory disorders (Kasprzak-Drozd et al., 2021). The use of plants for healing is an old approach that has persisted for years, and nowadays it receives special attention from consumers and researchers (Jurendić and Ščetar, 2021). Polyphenols are considered important natural antioxidants. Antioxidants are low molecular weight compounds that are of natural or synthetic origin and prevent or inhibit cell damage. They have the ability to scavenge free radicals that cause cell damage in the human body. Oxidative stress as a consequence of the action of free radicals leads to the development of different chronic diseases (Jurikova et al., 2017; Apak et al., 2016).
Black chokeberry ( Aronia melanocarpa) is a rich source of bioactive compounds with many functional activities that are mainly due to antioxidant activity of polyphenols. Chokeberry fruits have higher quantities of polyphenols than blackberries, blueberries, raspberries and others (Jakobek et al., 2007a). Chokeberry is very abundant in proanthocyanidins that are responsible for astringency and high antioxidant activity. Besides proanthocyanidins, anthocyanins, phenolic acids, flavonols and flavanols are present in chokeberries. For many years anthocyanins were used as natural food colorants. Nowadays, the focus of their application is as supplements and medicines. Anthocyanins in chokeberry are generally cyanidin glycosides. These found in significant concentrations are galactoside, glucoside, arabinoside, and xyloside. From the group of hydroxycinnamic acids, chlorogenic and neochlorogenic acids are present in the highest concentration. Also, quercetin derivatives are widely present (Willemse et al., 2013; Zhang et al., 2021; Zielińska et al., 2020).
The analysis of polyphenols is very challenging due to the great diversity and presence of conjugated forms. For the science community and food technology, the development of rapid, sensitive and accurate methods for polyphenols analysis is very important (Pyrzynska and Sentkowska, 2019). The high-performance liquid chromatography method was introduced in the 1970s and since then has been continuously used for the analysis of food compounds. The combination of liquid chromatography and mass spectrometry is suitable for the analysis of food samples with the advantages of separation chromatography and mass spectrometry as an identification tool . Mass spectrometry data can give information about type, molecular weight and number of glycosides (Motilva et al., 2013; Zhu et al., 2021).
This review addresses the main polyphenols present in chokeberries as well as their beneficial effects on human health. Also, the application of liquid chromatography and ultra-high liquid chromatography coupled with mass spectrometry or tandem mass spectrometry for qualitative and quantitative analysis of polyphenols are presented.
2. The main characteristics of chokeberries
Black chokeberry ( Aronia melanocarpa) belongs to the Rosaceae family native to North America. Besides Aronia melanocarpa there is also Aronia arbutifolia (red chokeberry) and a hybrid of black and red chokeberry, purple chokeberry ( Aronia prunifolia) (Jurendić and Ščetar, 2021). At the beginning of the 20th century, chokeberry was first mentioned in Northern, Eastern and Central Europe. Almost 90% of global chokeberries production belongs to Poland (Platonova et al., 2021).
Chokeberry shrubs grow up to 2-3 meters in height and have umbels of white flowers from which fruits ripen. They are very resistant to cold (they tolerate temperatures below -35 °C), so they can be grown in different climatic conditions (Jurendić and Ščetar, 2021). Chokeberry fruits primarily ripen in August, although they can begin to ripen as early as mid-July. It turned out that, for maximum fruit mass and anthocyanin content, it would be the most appropriate to harvest them at the beginning of September (Jurikova et al., 2017). The black chokeberry fruits have sweet, astringent and sour taste. Due to the unattractive taste of chokeberries, the food industry uses it for processing into juices, syrups, jams, jellies, fruit desserts, tea, puree, concentrates, fruit wine or natural food colorants (Esatbeyoglu et al., 2017; Sidor and Gramza-Michałowska, 2019). The chemical composition of chokeberries is connected with climate conditions, soil composition, harvest methods, maturity of berries and storage conditions (Jurendić and Ščetar, 2021). In Table 1., chemical composition of chokeberry fruits, juice and pomace are presented.
Table 1. Chemical composition of chokeberry fruits, pomace and juice (Jurendić and Ščetar, 2021)
3. Chokeberry polyphenols
Polyphenols are a large class of phytochemicals (over 8000 different compounds) widely represented in the plant kingdom (Ištuk et al., 2020; Pyrzynska and Sentkowska, 2019). The chemical structure of polyphenols is characterized by at least one aromatic ring with one or more hydroxyl groups attached (Motilva et al., 2013). They are present in plants seeds, leaves, fruits, roots and stems and are involved in the plant's defense system (Pyrzynska and Sentkowska, 2019). They are natural antioxidants and serve as UV screens, signaling molecules and antimicrobial agents. Polyphenols are mainly located in the outer layers of plant tissue and in seeds (Weber and Passon, 2019). Kaloudi et al. (2022) found out that around 30% of polyphenols of Aronia melanocarpa are located in the peel while the rest is present in the flesh and seeds. Generally, polyphenols are divided into subgroups such as simple polyphenols, phenolic acids, flavonoids, coumarins, stilbenes, condensed and hydrolysable tannins. Differences in the structure are the result of hydroxylation, acylation, alkylation and glycosylation reactions that change the basic molecule (Pyrzynska and Sentkowska, 2019). The most represented group of polyphenols in nature are flavonoids (over 4000 known flavonoids). Flavonoids are made of two aromatic rings connected by three carbon bridges (Ištuk et al., 2020; López-Fernández et al., 2020). According to the degree of polymerization and hydroxylation they can be divided into several subgroups: flavonols, flavanones, flavones, isoflavones, flavan-3-ols, anthocyanins and chalcones (López-Fernández et al., 2020). There is a diversity of plants that are abundant in flavonoids, such as vegetables, cereals, fruits, spices and herbs (López-Fernández et al., 2020). Polyphenols that are found in chokeberries belong to the groups of phenolic acids, anthocyanins, proanthocyanidins, flavonols and flavanols (Sidor and Gramza-Michałowska, 2019). The chemical structure of the main polyphenols identified in chokeberries is shown in Figure 1.
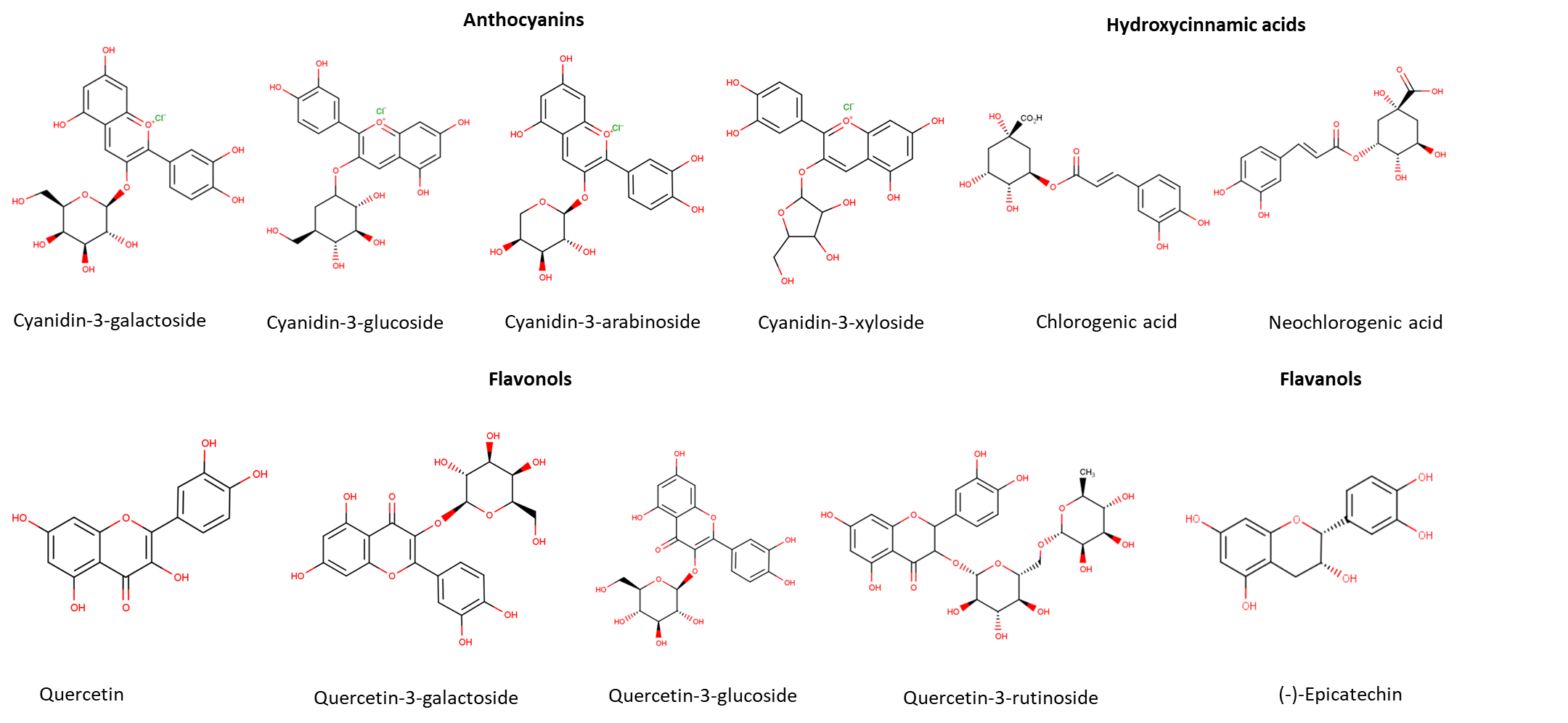
Figure 1. Chemical structure of the main polyphenols in chokeberries
Phenolic acids
Phenolic acids are made of a phenol ring with one or more hydroxyl groups attached and are divided into derivates of hydroxybenzoic acids and derivates of hydroxycinnamic acids (Rocha et al., 2012). Hydroxybenzoic acids derivates such as gallic, vanillic, syringic and salicylic acids are usually present in insoluble bound forms and make more complex compounds such as tannins and ligands. Their oncentration in fruits and vegetables are very low compared to hydroxycinnamic acids (Capriotti et al., 2014). Hydroxycinnamic acids have a simple C6-C3 chemical structure, and the main representatives are p-coumaric, ferulic, caffeic and sinapic acids. Although chemical structures are fairly similar, they have an impact on polyphenols properties and biological activity due to presence of hydroxyl groups on the aromatic ring. Hydroxycinnamic acids are rarely found in free form and more often as glycosylated derivatives of quinic, tartaric and shikimic acids (Coman and Vodnar, 2020). Chlorogenic acids are formed by esterification of cinnamic acids with quinic acid (Zielińska et al., 2020). Neochlorogenic acid is a chlorogenic acid isomer made by ester binding of caffeic and D-(-)-quinic acids. In chokeberries, the most abundant are chlorogenic acid (3-O-caffeoylquinic acid) and neochlorogenic acid (5-O-caffeoylquinic acid) (Zielińska et al., 2020).
Flavanols
Flavanols are the main subgroup of flavonoids present in black chokeberry including mainly (+)catechins and (-)epicatechins as well as proanthocyanidins condensed from them (Gao et al., 2023). Concentrations of proanthocyanidins in juice is very low, because they are abundant in pomace (Sójka et al., 2013). With the increase in the degree of polymerization, proanthocyanidins' functional and antioxidant activities decrease. Also, polymeric proanthocyanidins are poorly absorbed by the human body and the reason is in large molecular size and gut barrier. In this sense, the main contribution of polymeric proanthocyanidins to human health is reflected in the digestive tract, such as inhibition of amylase and lipase (Gao et al., 2023). Polymeric procyanidins represent 66% of chokeberry fruits polyphenols (Jurendić and Ščetar, 2021).
Anthocyanins
Anthocyanins are known as natural, widely spread water-soluble pigments. Due to their attractive color (red, blue, or purple), they are often used in food, pharmaceutical and cosmetics products (Płatosz et al., 2020). Anthocyanins are anhocyanidins (cyanidin, deplhinidin, malvidin, petunidin and peonidin) glycosylated derivates (Topić Božič et al., 2019). They are the second largest group of chokeberry polyphenols (Zhang et al., 2021) and are present in higher concentrations in pomace than in juice (Gao et al., 2023). Chokeberry anthocyanins are generally cyanidin glycosides (galactoside, glucoside, arabinoside and xyloside). Cyanidin-3-galactoside is the main anthocyanin in chokeberries. Galactoside and arabinoside make up more than 75% of total anthocyanins in chokeberries (Zhang et al., 2021; Wilkes et al., 2013) or more than 90% according to some authors (Klisurova et al., 2018). Cyanidin-3-galactoside and cyanidin-3-glucoside have very similar structures with the same molecular weight and the only difference is in the position of one OH group in the hexose ring. The same is for cyanidin-3-xyloside and cyanidin-3-arabinoside with the difference in OH position in the pentose ring (Esatbeyoglu et al., 2017).
Flavonols
Although flavonols are represented in small concentrations in chokeberry, they are widely studied due to their strong biological activity. Main flavonols in chokeberries are quercetin glycosides such as quercetin-3-O-rutinoside, quercetin-3-O-galactoside and quercetin-3-O-glucoside. Also, small concentrations of kaempferol, isorhamnetin, quercetin-3-O-robinobioside, eriodictyol-glucuronide have been found in the black chokeberry (Oszmiański and Lachowicz, 2016; Slimestad et al., 2005).
4. Health benefits of chokeberry
The high antioxidant capacity of chokeberry, i.e., its bioactive compounds, has made it the subject of numerous studies in the treatment of various chronic diseases as well as the prevention of their development. The results obtained in vitro in cells or cell lines, as well as in vivo on humans and animals, showed that chokeberry has a potential for the positive effect on the treatment of diabetes, cardiovascular diseases, and cancer, as well as antibacterial, gastroprotective, hepatoprotective and anti-inflammatory effects (Jurikova et al., 2017).
Inflammation is the response of the immune system to some undesirable stimuli in the body. Inflammatory reactions are accompanied by producing cytokines and they act as signals between immune cells (Kasprzak-Drozd et al., 2021). Chokeberry showed the possibility of inhibiting the release of cytokinome IL-6, IL-8 and TNF-α in the monocytes of humans (Appel et al., 2015; Jurikova et al., 2017; Kasprzak-Drozd et al., 2021). The study of Yamane et al. (2023) showed neuroprotective function of chokeberry juice on male 5XFAD Alzheimer’s disease model mice. Fraction that contained three quercetin glycosides reduced δ-secretase activity in SH-SY5Y cells (Yamane et al., 2023). Furthermore, chokeberry extract showed significant ability to reduce tissue damage in the hippocampus in mice (Lee et al., 2018). The reduction of monocytes and granulocytes levels, which cause inflammation in diabetic rats, as well as increase of lymphocytes, which inhibit atherosclerotic plaques, by chokeberry extract, is one of the proofs of the positive impact on cardiovascular diseases (Sidor et al., 2019). Some studies showed positive effects of chokeberries on hyperlipidemia and hypercholesterolemia. For example, chokeberry juice consumption caused a reduction of the high level of triacylglycerols in women (Nowak et al., 2016), while juice with glucomannan decreased the high-density lipoprotein levels and systolic blood pressure from 127.6 to 116.4 mmHg in obese women (Kardum et al., 2014). A positive impact on the lipid profile of chokeberries was noticed in obese patients and those with metabolic syndrome (Sidor et al., 2019). Laboratory studies on animals showed a reduction in glucose levels after chokeberry feeds in merino lambs (control group 3.38 mmol/L; 150 and 300 g chokeberry/kg feeds 2.42 and 1.55 mmol/L, respectively) (Lipińska and Jóźwik, 2017). Anthocyanins and their metabolites that are absorbed by the human body may have an impact on organs with the flow of blood. The unabsorbed anthocyanins have an impact on digestive tract on enzymes, intestinal flora and epidermal cells (Gao et al., 2023). Given that cyanidin glycosides are poorly adsorbed in the human body, the influence of chokeberry anthocyanins is mainly based on the intestinal microflora (Yu et al., 2021; Gao et al., 2023). On the other hand, Płatosz et al. (2020) found out that chokeberry anthocyanins adsorb and appear in the body fluids of sheep, but over 70% as metabolites. Anthocyanin levels in cerebrospinal fluid ranged from 0.04-0.32 µmol/L, but future studies need to explain mechanism of anthocyanins transfer to the brain and if these levels may have biological activity. Previous research showed that anthocyanins from chokeberry may be beneficial in decreasing blood glucose levels through α-glucosidase inhibition, relieving oxidative stress (Bräunlich et al., 2013) and have hypolipidemic effects (Kim et al., 2013). Cyanidin-3-arabinoside has high inhibitory activity toward α-glucosidase and together with proanthocyanidins may be responsible for diabetes prevention (Kaloudi et al., 2022). Also, black chokeberries possess the potential to inhibit the development of leukemia, intestinal cancer and breast cancer (Sidor et al., 2019).
There are a lot of comprehensive reviews of chokeberry health benefits (Kasprzak-Drozd et al., 2021; Hawkins et al., 2020). Gao et al. (2023) published a review of chokeberry polyphenols and their impact on hypertension, hyperlipidemia, obesity, hyperglycemia and inflammation. Also, a comprehensive review by Sidor et al. (2019) describes health promoting effects of chokeberry and its products.
5. Liquid chromatography for polyphenols analysis
Sample preparation
Before the analysis, adequate sample preparation is obligatory. For liquid samples, filtration or centrifugation before analysis is enough. For solid samples, several types of pretreatments (sieving, milling, grinding, and homogenization) are necessary (Pyrzynska and Sentkowska, 2019). Studies showed that grinding samples before solvent extraction resulted in better polyphenols accessibility than ungrounded samples (Pyrzynska and Sentkowska, 2019; Becker et al., 2016). It has been shown that dry, freeze-dried, or frozen samples, that are in powder form, are better than fresh material due to possible degradation in the presence of enzymes, especially when long-term solvent extractions are performed. Since most of the polyphenols are linked to the cell wall, for effective extraction it is necessary to disrupt the structure and allow the release of those compounds (Capriotti et al., 2014).
For liquid chromatography-based methods, solvent extraction methods are necessary (Capriotti et al., 2014). Solvent extraction of the analytes depends on the sample nature and chemical properties of polyphenols such as molecular structure and polarity (Mojzer et al., 2016). Usually used solvents are methanol, acetone, ethanol, ethyl acetate, or their mixture with water. A certain amount of water in the mixture is needed due to the high polarity of some polyphenols such as phenolic acids (Capriotti et al., 2014). Since anthocyanins are more stable at low pH, aqueous-organic solvents with acidic pH are usually used. It should be noted that there is a lack of solvents that can fully extract all compounds of interest (Pyrzynska and Sentkowska, 2019). Hydrolysis treatment of bounded polyphenols to the cell wall (polysaccharides, lignins, or proteins) or polyphenols trapped in cores is often. Hydrolysis can be acid, alkaline, or enzymatic and is a very challenging procedure. To avoid the oxidation of polyphenols, an inert atmosphere for hydrolysis is used (Capriotti et al., 2014). For polyphenols extraction, conventional methods, ultrasound-assisted and microwave-assisted extraction methods are usually used (Rajbhar et al., 2014).
High-performance liquid chromatography (HPLC), ultra-high performance liquid chromatography (UHPLC) and comprehensive two-dimensional liquid chromatography (LCxLC)
The analysis of polyphenols is very demanding due to the great diversity and presence of conjugated forms. Previously used methods for polyphenols purification and identification were thin-layer chromatography and column chromatography (Ajila et al., 2010). For the science community and food technology, the development of rapid, sensitive and accurate methods for polyphenols analysis is very important (Pyrzynska and Sentkowska, 2019). The commonly used technique for polyphenols determination is UV-Vis spectroscopy. The disadvantage of this method is an inability to identify individual polyphenols (López-Fernández et al., 2020). To overcome that issue, high-pressure liquid chromatography (HPLC), usually equipped with a diode array detector (DAD), is used for the separation of these compounds.
The limitation of this detector is that the identification of compounds is achieved only by UV-Vis spectra and retention time (Pyrzynska and Sentkowska, 2019; López-Fernández et al., 2020). Despite that, the HPLC method is preferred for the identification and quantification of polyphenols. Some of the commonly used HPLC method requirements are a reverse phase C18 column, a UV-Vis diode array detector, and a binary solvent system where the solvents are usually acidified water and a polar organic solvent (Ignat et al., 2011). Since HPLC analysis requires long analysis time and high resolution for complex samples and speed is an important factor in laboratories, ultra-high performance liquid chromatography (UHPLC) has been developed. With this kind of improvement, it became possible to achieve 5-to-10-fold faster separation with a small detection volume. The use of shorter columns with smaller particle sizes minimizes the injection volume and increases mobile phase linear velocity. So, the advantages of UHPLC over HPLC are improved resolution, faster separation, higher peak efficiency, and reduced solvent consumption (Motilva et al., 2013). Polyphenols are generally small molecules and for their separation, it is necessary to use columns with small pores to avoid the leak of molecules and ensure interaction with analytes (Tong et al., 2021).
Together with columns with small particles, UHPLC systems with up to 1300 bar working pressure ensure improved separation speed and peak efficiency (Capriotti et al., 2014). Due to the rapid separation of UHPLC, the mass spectrometer (MS) must be connected to ensure an equally fast-duty cycle (Motilva et al., 2013). Comprehensive two-dimensional liquid chromatography (LCxLC) ensures better resolution in determination of polyphenols present in a complex matrix. In this technique, the on-line transferring of all fractions from the first to the second dimension occurs (Motilva et al., 2013; Motilva et al., 2013). Compounds separated in the first dimension stay separated in the second dimension with the preservation of elution profiles of both dimensions (Dugo et al., 2008).
Detectors
For the identification of polyphenols, different detectors have been used together with HPLC separation such as UV-Vis and DAD, fluorescence, chemiluminescence, or mass spectrometry detectors. Nowadays, MS and diode array detectors are used mostly. Liquid chromatography, coupled to tandem mass spectrometry (LC-MS/MS), is an advanced technique used nowadays to overcome all the shortcomings of the previously mentioned techniques (Pyrzynska and Sentkowska, 2019; López-Fernández et al., 2020). The combination of LC and MS is suitable for the analysis of food samples with the advantages of separation chromatography and MS as an identification tool (Motilva et al., 2013). Mass spectrometry data can give information about type, molecular weight and number of glycosides (Zhu et al., 2021). The occurrence of chromatographic columns packed with sub-2 µm particles and mass spectrometry with high resolution made the analyses of polyphenols from complex matrix much easier (Motilva et al., 2013). For the analysis with MS detector, several types of ionization in the ion source may be used. The most common are electrospray ionization and atmospheric pressure chemical ionization (Pyrzynska and Sentkowska, 2019). Some other MS systems are fast atom bombardment mass spectrometry, MS-MS, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and electron impact mass spectrometry (Ajila et al., 2010).
Electrospray ionization is very favorable for the identification of polar compounds that are separated by liquid chromatography. This is a gentle ionization method that generates deprotonated molecules monitored in positive or negative mode (Ajila et al., 2010; Pyrzynska and Sentkowska, 2019). Negative mode is very useful for the compounds in the matrix due to higher selectivity (Pyrzynska and Sentkowska, 2019). Positive mode is generally used for anthocyanins (Karaaslan and Yaman, 2016). A group of authors proposed negative ionization as an effective way for anthocyanins identification. They pointed out the problem of interfering with some flavonol glycosides and anthocyanins ions and fragmentation patterns in positive ionization. In negative mode, doublet ions of [M-2H]- and [M-2H +H2O]- are only for anthocyanins (Sun et al., 2012).
Time-of-flight mass spectrometry provides a measurement of the isotopic pattern, helping determine the elemental composition of the compounds. MS-MS analysis provides information of aglycone moiety, carbohydrate types or present substituents, the sequence of glycan part, stereochemical assignment of terminal monosaccharide units, interglycosidic linkages and the attachment points of the substituent to the aglycans (Ajila et al., 2010; Cuyckens and Claeys, 2004).
6. Liquid-chromatography for identification and quantification of chokeberry polyphenols
Anthocyanins have the ability to absorb in the visible range (about 500-550 nm), which makes them selectively detected and differentiates them from other groups of flavonoids. Liquid chromatography coupled with the photodiode-array detector, has limitations in the identification of individual anthocyanins because it does not provide enough structural information (Willemse et al., 2013). Reverse phase liquid chromatography with mass spectrometry gives information on molecular mass and structure and makes easier the identification of anthocyanins. Furthermore, tandem mass spectrometry (MS/MS) provides additional information due to selective fragmentation.
Limitations of LC-MS of anthocyanins identification in mixtures occur due to identical molecular ions and fragments between glycosidic isomers such as galactosides and glucosides. For these issues chromatographic separation is crucial. To overcome low chromatographic efficiency, it is necessary to use the gradient program that lasts for a longer period of time (Willemse et al., 2013). Furthermore, increasing the temperature during analysis improves chromatographic separation through the reduction in band broadening and better peak shape and resolution is achieved. It was observed that temperatures up to 50 °C do not cause thermal degradation of anthocyanins for analysis less than one hour (De Villiers et al., 2009).
Meng et al. (2019) studied anthocyanins from Aronia melanocarpa from China. They perform purification of anthocyanins with absorbent resin and identified seven anthocyanins (cyanidin-3,5-dihexoside, dimer of cyanidin-hexoside, cyanidin-3-galactoside, cyanidin-3-glucoside, cyanidin-3-arabinoside, cyanidin-3-xyloside, and delphidinin-3-rutinoside) by HPLC and mass spectrometry. Cyanidin-3,5-dihexoside and dimer of cyanidin-hexoside were found for the first time in Aronia melanocarpa (Meng et al., 2019). Furthermore, group of authors developed a HPLC-MS/MS method for identification and quantification of chokeberry anthocyanins (cyanidin-3-galactoside, cyanidin-3-glucoside, cyandind-3-arabinoside, and cyanidin-3-xyloside) as well as their metabolites in rat plasm, urine and feces. For chromatographic separation they used Poroshell EC-C18 column and for elution formic acid (0.1%) in methanol and formic acid (0.1%) in water. The optimized flow rate was 0.3 mL/min and temperature of 30 °C.
Mass spectra were obtained by scanning a mass range of m/z 100-2000 Da (Tong et al., 2021). Romani et al. (2016) investigated polyphenols and volatile compounds in commercially available chokeberry products (compote, juice and dried fruit). Using HPLC-TOF analysis authors identified, among other known polyphenols, a 4-O-caffeoyl-quinoic acid based on retention time in comparison with isomers and fragments in negative ionization mode. The main fragment ion was at m/z 353 and minor ion fragments were at m/z 191 and m/z 173. The ion at m/z 173 (“dehydrate” quinic acid moiety) is typical for a substituted isomer in position 4. Table 2 gives molecular ions and fragment ions of polyphenols commonly present in chokeberries. By reviewing the literature, the compounds listed in Table 2 were mostly identified and quantified in chokeberry fruits, juice, or pomace. Table 3 provides an overview of several researches conducted on chokeberry polyphenols as well as details of used methods, extraction techniques and main observations.
Table 2. LC-MS data of main polyphenols present in chokeberries (Rodríguez-Werner et al., 2019)
Table 3. Selected studies of chokeberry polyphenols analyzed by liquid chromatography
7. Conclusion
Polyphenols are known as antioxidants that effectively remove free radicals and have an impact on prevention and treatment of chronic diseases. Extensive research has shown that some polyphenols have a direct effect on individual human tissues and/or organs and can thus have a potential to positively influence the course of disease development. Chokeberries are abundant in polyphenols, what makes them the subject of many studies. Liquid chromatography coupled with mass spectrometry is the most powerful technique for identification and quantification of polyphenols. With the information that this review provides, the scientist can easily get insight into the main parameters for chokeberry polyphenols analysis, such as solvents for chromatographic elution, preferred columns for separation, detectors, as well as polyphenols profile of chokeberries.
Author Contributions: Conceptualization, L.J., D.K., M.K. and J.Š.; methodology, I.B., L.J., D.K. and M.K.; investigation, I.B., L.J., D.K. and A.P.; data curation, I.B., L.J., D.K. and A.P.; writing—original draft preparation, I.B..; writing—review and editing, LJ., D.K., M.K. and JŠ.; funding acquisition, M.K. and J.Š. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by project IP-2019-04-5749 (which was fully supported by the Croatian Science Foundation) and PZS-2019-02-1595 (which was fully supported by the “Research Cooperability” Program of the Croatian Science Foundation and funded by the European Union’s European Social Fund under the Operational Program for Efficient Human Resources 2014–2020) and project.
Conflicts of Interest: The authors declare no conflict of interest.
