Introduction
Bryophytes, like other plants, suffer from environmental changes and habitat destruction (Löbel et al. 2018). Rapid climate alternations have led to a decrease in bryophyte populations despite their dispersal potential (Zanatta et al. 2020), especially those living at the edge of their overall ranges. These facts along with their fast reaction to small changes (good indicators) make this group of plants very vulnerable and susceptible to high extinction risks both regionally and globally. Although bryophytes are included in general and national conservation initiatives (Hallingbäck and Tan 2010), active protection is rarely applied (Rowntree et al. 2011, Sabovljević et al. 2014a), especially long-term. Another problem is the lack of knowledge concerning the biology and ecology of rare and threatened bryophyte species, which makes it rather difficult to apply active measures prior to thorough studies. Recently, in order to overcome these problems, the emerging discipline of conservation physiology has begun to address species protection, offering the possibility to study species outside their natural populations and habitats and to apply new knowledge to their propagation and species protection. Pioneering work on bryophytes in this direction has been conducted recently (Sabovljević et al. 2022).
Entosthodon pulchellus (H. Philip.) Brugués is a funaroid moss species found in areas of the sub-Mediterranean-sub-Atlantic regions of Europe, and is also known from North Africa, South-west, central and eastern Asia. Although it is rarely reported, Hodgetts et al. (2019a,b) consider its overall European population stable, while the range-edge populations seem to be declining, like those in Germany. The same authors also reported the populations to be extremely rare in Austria and Switzerland, and stable in Hungary and Portugal.
The species is known to inhabit thin soil on calcareous terraces, with scarce vegetation and has an ephemeral and shuttle life strategy. Sporophytes are often present since the species is autoicous. According to known reports it is altitudinally rather indifferent, also appearing in sunny and warm places in high mountains, usually in spring to early summer when it completes its short life cycle, culminating in spore dispersal.
Priority in conservation management is given to more rare genotypes and subpopulations (e.g. Jin et al. 2020). Thus, in this study we have focused on the Russian accession of E. pulchellus with the aim of establishing an ex situ population to learn more about its biology and to optimise captive propagation and large-scale biomass production. This should enable reintroduction to potentially suitable habitats and/or population strengthening in declining subpopulations. Additionally, since the species from this genus often hybridise naturally (Ostendorf et al. 2021) and the ploidy status is unclear throughout its populations, this approach will provide clean material for further studies.
In Russia this species is known mainly from its Asian part, including southern Siberia (the Altai Republic, Zabaikalsky Territory, the Republic of Buryatia), the Amur Province in the Russian Far East, and Yakutia. It grows at altitudes ranging from 100 to 1650 m a.s.l. in various habitats, such as the niches and cracks of rock outcrops, screes, and on bare soil on steep slopes with steppe vegetation (the latter kind of habitat is sporadic in Yakutia, occurring up to 68° N). However, in European Russia E. pulchellus is much rarer, found only in xeric SE regions in the Astrakhan and Volgograd Provinces, where it grows on the slopes of ravines and karst sinkholes, in slightly wetter conditions than E. hungaricus (Boros) Loeske which appears not to be rare on soil among grasses in steppe communities.
The species is critically endangered (IUCN: CR) in Switzerland, endangered (IUCN: EN) in Slovenia, Czechia, Germany and Ireland, and at serious risk of extinction in Austria (Hodgetts et al. 2019b, Martinčič 2024). In Britain and Hungary, it is considered near threatened (IUCN: NT), although rare but stable (Hodgetts et al. 2019b), while in many other countries it is a data deficient species (IUCN: DD) due to its rarity, ephemeral life span and/or under-recording (e.g. Croatia, Serbia, and Albania).
Plant material
The plant material for the present study was collected in the Altai Republic, Ulagan District, on the left bank of the Chulyshman River, 1 km downstream, at the Chulcha Creek mouth, 51°5'32" N – 87°58'51" E, altitude 520 m a.s.l., in Rhododendron L. thickets on a steep mossy slope, on the soil ledge, 17 June 2021, coll. M.S. Ignatov & E.A. Ignatova #21-251 (MW9092068, MHA9130772). Herbaria acronyms follow Thiers (2024).
Sporophytes from the deposited herbarium material were used to establish the in vitro culture of E. pulchellus. Mature sporophyte capsules were carefully separated from the dry plant material, thoroughly cleaned of mechanical impurities and rinsed in distilled water. Then the cleaned capsules were sterilised with 7% sodium hypochlorite solution (NaOCl). In addition to the different NaOCl concentrations, the exposure time was also varied, with durations up to 120 sec to obtain uncontaminated spores prior to their dispersal into the KNOP basal medium under the flow chamber as previously elaborated (e.g. Jadranin et al. 2023). After the spores were spread on the medium, spore germinability and the presence of possible contamination by remnants of the cohabitants were tested and then subcultivated to fresh medium until a fully axenic culture was achieved. The Petri dishes containing the spores were cultured for two months under sterile conditions at a constant temperature (18 ± 2 °C) and humidity (60 - 70%) in a long-day light regime (16 h light/8 h dark). Light with a flux density of 50 µmol m-2s-1 was provided by fluorescent tubes (Tesla Pančevo, Serbia). The axenic cultures thus obtained were subsequently micropropagated and used as starting material for the experiments presented here.
In vitro micropropagation of the plant material
After the achievement of axenic plantlets, the plants were propagated for six weeks on a minimal KNOP nutrient medium (Reski and Abel 1985) until the development of gametophores and their optimal size and biomass for the experimental treatments had been achieved. For further investigation, the explants were placed in Petri dishes with the appropriate media type depending on the experiment. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C for 45 min. A single gametophore of 5 mm in length was used as the starting explant in all the treatments. Each experimental group consisted of 20 individual gametophores. The experiment was performed axenically under controlled conditions as described in the plant material chapter.
Experimental design
In this study, two different experiments were conducted. Experiment type I was used to investigate the effects of nutrient media and exogenously added sugars on the morphogenesis of E. pulchellus. The explants were grown on a minimal KNOP medium (Reski and Abel 1985), a Murashige and Skoog (MS) half-strength medium (designated MS/2) (Sabovljević et al. 2009), and a BCD medium (Sabovljević et al. 2009) to which 15 g L-1 of sucrose or fructose were subsequently added. The concentrations of sucrose and fructose were used at half-strength; sugars are usually added at full strength (30 g L-1) in media for vascular plant cultivation (see Tab. 1 for details).
Tab. 1. Experimental design summary.
Since bryophytes grow predominantly photoautotrophically compared to photomixotrophic vascular plants under axenic conditions, the addition of a lower sugar concentration seemed to be more suitable for mosses, as shown in previous studies (e.g. Sabovljević et al. 2005).
In experiment type II, the effects of exogenous plant growth regulators (PGRs) on the morphogenesis of E. pulchellus were investigated. The plants were grown on a KNOP minimal medium supplemented with different concentrations of indole-3-butyric acid (IBA) and 6-benzylaminopurine (BAP) both individually and combined. The concentrations of IBA and BAP used in this experiment are listed in Tab. 1.
Morphological parameters such as the index of multiplication and the diameter of the secondary protonema were measured after four weeks. The index of multiplication (IM) represents the newly formed shoots which emerge from the new buds of the initial explant. All morphological changes were carefully measured and documented using a Leica stereomicroscope (Leica MZ 7.5 Bi-Optic Inc. Santa Clara, CA, USA) and a conventional light microscope (Leica DMLS, Santa Clara, CA, USA).
Statistical analysis
Statistical analysis was conducted using the R programming language (v. 4.3.1) (R Core Team 2022). The preliminary data exploration was done using the Shapiro-Wilk normality test and Levene's test of homogeneity of variance. Such analysis revealed that nonparametric statistics should be applied. Therefore, the Kruskal-Wallis rank sum test was used for a comparison of the experimental groups, after which Dunn's multiple comparison test with the Benjamini-Hochberg P-value adjustment method was applied, with one exception, in experiment type II for the comparison of the diameters of the secondary protonema patches, where the Wilcoxson rank-sum test was used in order to compare the two groups. The significance level (α) was set at 0.05 for all experiment.
Results
The influence of different growth medium types and exogenously added sugars on the morphogenesis of Entosthodon pulchellus
In experiment type I, the largest number of newly formed gametophores was documented in those plants grown on the sugar-free MS/2 medium (Fig. 1A). Numerous shoots were also developed in the plants grown on the KNOP medium with exogenously added sucrose (KNOP+S) and the sugar-free KNOP medium (Fig. 1A).
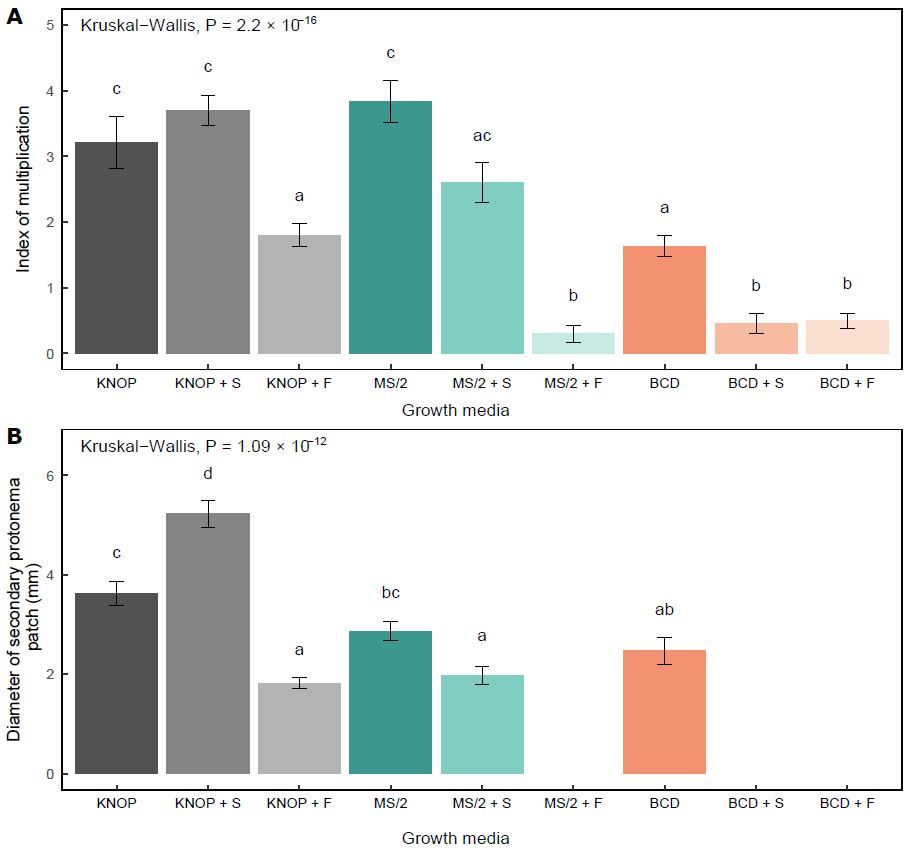
Fig. 1. The influence of the plant growth medium types and exogenously added sugars on the index of multiplication (A) and diameter of secondary protonema patch (B) in experiment type I. The data are presented as the mean ± standard error. Different letters above the bars denote statistically significant differences (P < 0.05) between the experimental groups.
However, no statistically significant differences were observed between these three experimental groups (MS/2, KNOP, and KNOP+S), suggesting that these media may be equally suitable for the in vitro cultivation of the species. Compared to the KNOP and MS/2 sugar-free media, IM was significantly lower in the plants grown on the BCD medium (P < 0.05) (Fig. 1A).
In general, the addition of sugars negatively affected the formation of new buds and shoots in E. pulchellus, except when sucrose was combined with the minimal KNOP medium (KNOP+S). When fructose was added to the medium, the IM decreased significantly in all three types of media used in experiment type I (P < 0.05) (Fig. 1A). A similar effect was observed when the BCD medium was supplemented with sucrose (BCD+S) at the same concentration (15 g L-1).
As for the development of the secondary protonema patch, a similar pattern was recorded, i.e. those plants grown on sugar-free media developed regular protonemal patches although different in size (Fig. 1B). The largest diameter of secondary protonema was observed in the plants grown on the KNOP medium containing 15 g L-1 sucrose (KNOP+S) (Fig. 1B). However, the addition of sucrose exerted different effects on the development of the secondary protonema depending on the growth media type. Although sucrose had a positive effect on protonemal development in the plants grown on the KNOP+S medium (P < 0.05), when added to the MS/2 medium, sucrose negatively affected the protonemal growth (P < 0.05). Furthermore, sucrose completely inhibited the development of secondary protonema when present in the BCD medium (BCD+S) (Fig. 1B), i.e. no plants developed measurable protonemal patches. On the other hand, fructose significantly decreased the protonemal diameter in E. pulchellus when added to the KNOP medium (KNOP+F) (P < 0.05), while it completely inhibited the formation of protonemata in the plants grown on the MS/2 and BCD media at a concentration of 15 g L-1 (Fig. 1B).
In addition to the evaluation of the two morphogenesis parameters, the morphological appearance of the plants was also taken into account in order to determine the optimal medium for the in vitro micropropagation of E. pulchellus (Fig. 2).

Fig. 2. The appearance of Entosthodon pulchellus explants grown on sugar-free media (A-C) and media supplemented with 15 mg L-1 sucrose (D-F) and 15 mg L-1 fructose (G-I) in experiment type I. The bars represent the size of 2 mm in accordance with the magnification (0.63× for A-C, F and G; 0.8× for D and E; 1× for H; 1.6× for I).
Overall, according to the results of experiment type I, the sugar-free KNOP and MS/2 media appeared to be equally suitable for the in vitro micropropagation of the investigated species (Fig. 2A, 2B). However, since the addition of sucrose to the KNOP medium promoted the development of protonemata, this medium could be used for the purpose of growing more protonemata than shoots (Fig. 2D), and for easier and rapid multiplication. Compared to the KNOP and MS/2 media, the BCD medium was generally inadequate for the micropropagation of E. pulchellus, as the plants did not develop a sufficient number of new shoots and secondary protonema patches (Fig. 2C, 2F and 2I).
An interesting phenomenon was observed in those plants grown on the fructose-enriched MS/2 medium (MS/2+F) (Fig. 3). The phylloid cells underwent dedifferentiation and multiple secondary protonemal threads emerged from the individual cells (shown by arrows in Fig. 3), indicating possible sublethal conditions for E. pulchellus upon addition of fructose to the MS/2 medium.

Fig. 3. The formation of secondary protonemal threads from phylloid cells (arrows) in Entoshtodon pulchellus explants grown on MS/2 medium supplemented with fructose. Scale bar = 200 µm (magnification 10×).
The influence of the plant growth regulators on the morphogenesis of Entosthodon pulchellus
To assess the effects of the plant growth regulators, the plants were grown on the KNOP medium supplemented with different concentrations of IBA and BAP as described in the Material and Methods section (Tab. 1). The minimal KNOP medium was selected according to the results obtained in experiment type I as the most suitable minimal medium for the propagation of the examined species.
The highest IM was recorded in the plants grown on the KNOP medium without exogenously added PGRs (control experimental group), although a large number of small shoots were also observed in the plants grown on the medium supplemented with 0.1 μM BAP or 0.1 μM IBA (Fig. 4A).
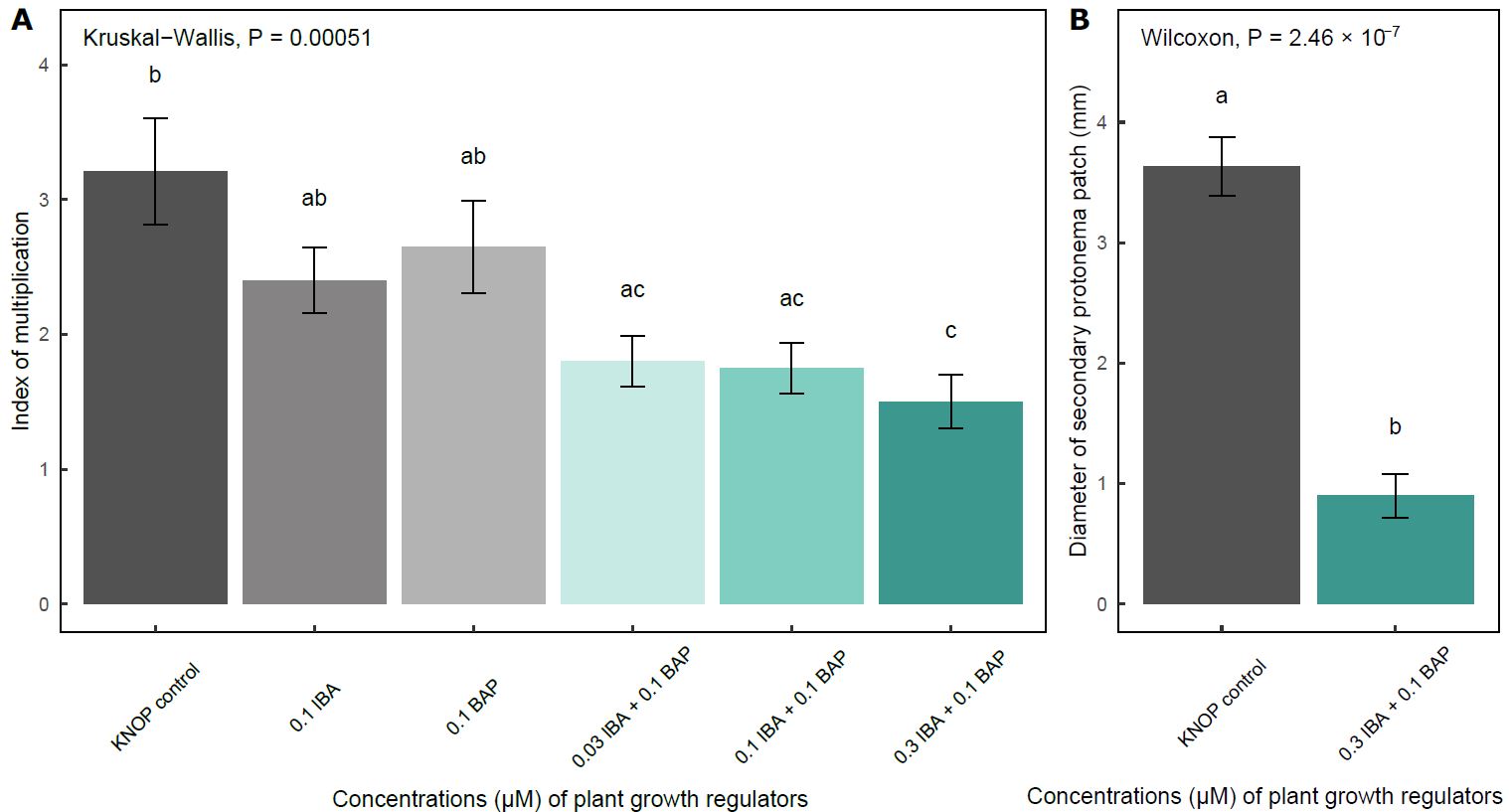
Fig. 4. The effect of KNOP growth media supplementation with the plant growth regulators on the index of multiplication (A) and diameter of secondary protonema patch (B) in experiment type II. The data are presented as the mean ± standard error. Different letters above the bars denote statistically significant differences (P < 0.05) between the experimental groups.
It is important to note that there were no statistically significant differences between those three experimental groups, i.e. between the control plants and the plants grown on the KNOP medium enriched with IBA and BAP separately. However, when combinations of IBA and BAP were applied, there was a significant decrease in IM compared to the control group (P < 0.05), which was especially observed in those plants grown on the KNOP medium supplemented with the highest concentration of IBA (0.3 μM) in combination with 0.1 μM BAP (Fig. 4A). These results may indicate that the potential synergistic effect of the two PGRs exerted a negative impact on the formation of new shoots in the examined species.
A peculiar situation was observed regarding the development of secondary protonemal patches in experiment type II. Protonemata were recorded in only two experimental groups, i.e. the plants developed measurable protonemal patches (Fig. 4B). Specifically, such protonemal patches were documented in the control group and in those plants grown on the medium supplemented with 0.3 μM IBA and 0.1 μM BAP, with the latter experimental group exhibiting a significantly smaller diameter of secondary protonema (P < 0.05) (Fig. 4B).
The plants grown on the KNOP media supplemented with PGRs developed normal phylloids and protonema, albeit smaller than the plants in the control group (Fig. 5). The formation of small buds which did not develop into long shoots were documented in the plants grown on the medium with a combination of IBA and BAP (Fig. 5D-5F).
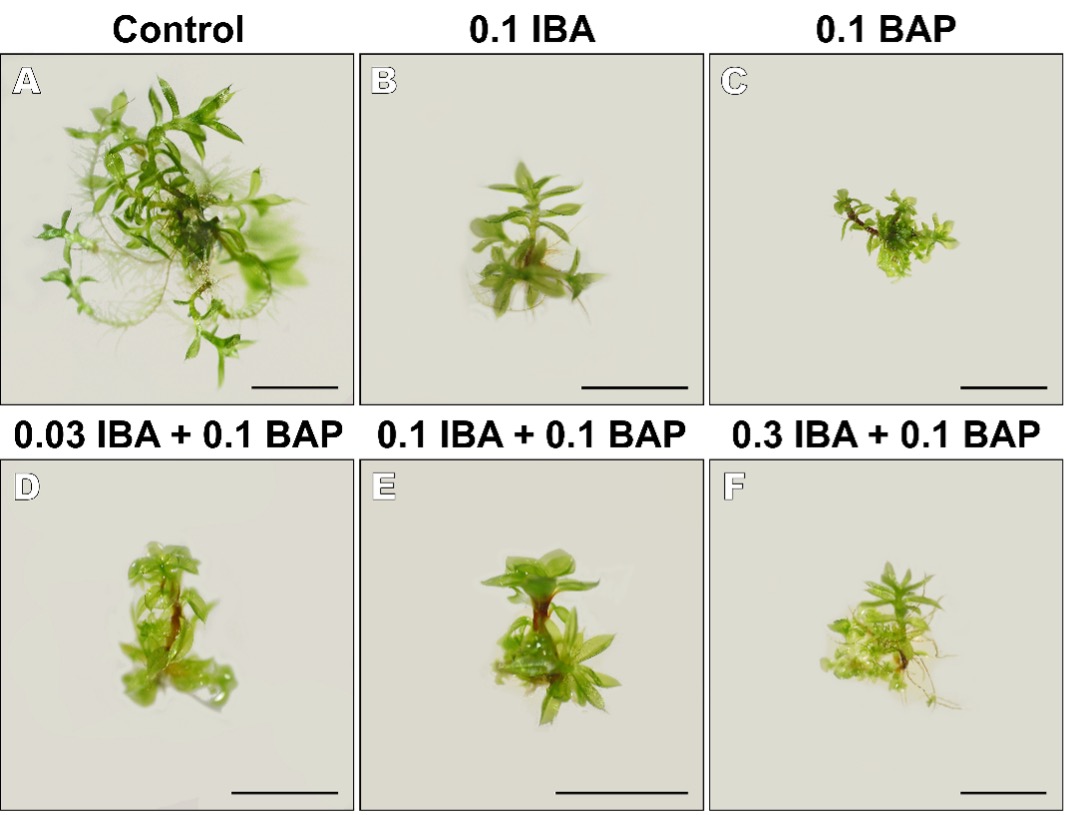
Fig. 5. The appearance of Entosthodon pulchellus explants grown on PGR-free KNOP media (A) and KNOP media supplemented with 0.1 μM IBA (B) and 0.1 μM BAP (C) individually or combined (D-F) in experiment type II. The bars represent the size of 2 mm in accordance with the magnification (0.63× for A, C and F; 0.8× for B and D; 1× for E).
Conversely, measurable secondary protonema only formed when IBA was used at a higher concentration than BAP (Fig. 4B and Fig. 5F). Overall, these results indicate that the applied concentrations of PGRs were not necessary for the micropropagation of E. pulchellus and that this species developed better in the absence of exogenously added IBA and BAP.
Discussion
The influence of different growth medium types and exogenously added sugars on the morphogenesis of Entosthodon pulchellus
In order to establish the conditions for the micropropagation of E. pulchellus, three different types of media were used in this study. According to the results obtained in experiment type I, both the MS/2 and KNOP media are equally suitable for long-term propagation (Fig. 1 and Fig. 2). In addition, the sucrose-enriched KNOP medium (KNOP+S) can also be used for the massive biomass production of secondary protonema, thus contributing to the vegetative growth and multiplication of this species in vitro. On the other hand, the BCD medium had negative effects on the development of shoots and protonemal filaments both when it was used sugar-free (Fig. 1A and Fig. 2C) and when enriched with sucrose (BCD+S) or fructose (BCD+F) (Fig. 1A and Fig. 2F, 2I). One of the possible reasons for such results may be the composition of the growth media used. Unlike the KNOP and half-strength MS media, the BCD medium contains potassium nitrate (KNO3) in a rather high concentration, which may affect the development of E. pulchellus. However, the BCD medium has been shown to be suitable for the micropropagation of certain species in vitro, such as the bryo-halophyte Hennediella heimii (Hedw.) R.H.Zander (Ćosić et al. 2022), Molendoa hornschuchiana (Hook.) Lindb. ex Limpr. (Vujičić et al. 2012), and Thamnobryum alopecurum (Hedw.) Gangulee (Sabovljević et al. 2012a). In addition, the bryo-halophyte Entosthodon hungaricus (Boros) Loeske formed more numerous new buds when cultured on a BCD medium than on a MS medium, although it did not form large patches of secondary protonema (Sabovljević et al. 2012b). Therefore, BCD is one of the common media types used in experiments for the in vitro propagation of mosses, but remains a rather peculiar one. Half-strength MS and KNOP minimal media, on the other hand, contain all essential nutrients in optimal amounts and are more suitable for bryophytic growth as bryophytes often live on mineral-poor substrates and do not have high nutrient requirements, nor do they absorb very much from the substrate in natural conditions.
In addition, numerous studies to date have shown that certain types of media are more suitable for the cultivation of mosses in vitro and that some species develop better on solidified media without sugars or PGRs (e.g. Sabovljević et al. 2022, Jadranin et al. 2023). In this study, the results clearly indicate that the addition of sugar was not necessary to promote the growth of E. pulchellus (Fig. 1 and Fig. 2), and that the addition of fructose in combination with BCD media can be detrimental (Fig. 5). In fact, the plants propagated better on sugar-free media, except in the case when sucrose was added in the minimal KNOP medium (KNOP+S) (Fig. 1 and Fig. 2). Stimulatory effects of moderate sucrose concentrations have already been documented for several moss species such as Dicranum scoparium Hedw. (Vujičić et al. 2009), Atrichum undulatum (Hedw.) P. Beauv. (Sabovljević et al. 2005), and Pogonatum urnigerum (Hedw.) P. Beauv. (Cvetić et al. 2007). In addition, some species did not develop buds in the absence of sucrose in the medium, such as Leptobryum pyriforme (Hedw.) Wilson and Barbula gregaria (Mitt.) A. Jaeger (Mitra & Allsopp 1959). Besides sucrose, other sugars have also been frequently used as stimulants for developmental processes. In Bryum argenteum Hedw., for example, fructose had a positive effect on the growth of protonemal patches (Bijelović et al. 2004) and on the formation of sex organs (Sabovljević et al. 2005) when applied in low to moderate concentrations. Sugars such as glucose, fructose and sucrose are essential components of plant metabolism and serve as energy suppliers (Hassid and Putman 1950). Although they are necessary for plant growth and development and play a role in responses to environmental stresses (Klavina 2014), high sugar concentrations may have negative effects on bryophyte growth and development in vitro since bryophytes are rather autotrophic in axenic conditions and do not require additional carbon sources for proper vegetative growth like tracheophytes (Sabovljević et al. 2022). In this study, fructose in combination with the MS/2 medium (MS/2+F) caused the occurrence of an interesting phenomenon (Fig. 3). The plantlets grown under these conditions showed a high degree of dedifferentiation of thread cells of secondary protonemata from which the new protonemal filaments emerged. This response to unfavourable conditions was previously documented for the phylogenetically related species E. hungaricus grown on a KNOP medium supplemented with 250 mM NaCl (unpublished data). It was hypothesised that this might be the strategy used by funaroid mosses to avoid conditions similar to osmotic stress which fructose could promote in combination with other compounds from MS medium. Nevertheless, there is a wide range of data in the literature on the influence of exogenous sugars on the formation of new shoots and buds, suggesting a species-specific response to additional carbon sources directly related to specific nutritional requirements and developmental strategies. According to the contradictory results in the literature, the results obtained for E. pulchellus are not unexpected, but further studies with more sugar species and a wider range of concentrations are needed to clarify their role in developmental processes in axenic conditions. Thus, the effects of sugars remain obscure both generally and specifically and they could have multiple roles in bryophytes when externally added, such as serving as a carbon source, or playing signalling, structural or functional roles.
The influence of the plant growth regulators on the morphogenesis of Entosthodon pulchellus
Exogenously applied PGRs have different effects on developmental processes in bryophytes, as shown by studies conducted to date on various moss species (Sabovljević et al. 2014b, 2022). Some species develop spontaneously in axenic conditions, such as Physcomitrella patens (von Schwartzenberg 2009), while other species require exogenous PGRs for the induction of secondary protonema and bud formation. However, there is still a lack of data on how synthetic auxins and cytokinins in combination affect the morphogenesis of bryophytes and what the optimal concentration range is for the initiation and development of specific structures in bryophytes (Jadranin et al. 2023). This information is crucial for understanding the developmental process and requirements of bryophytes in vitro to ensure the optimal micropropagation and conservation of endangered and rare species.
In this study, the effects of IBA and BAP, applied both individually and in combination, on the vegetative growth and development of E. pulchellus were investigated (Fig. 4A, 4B). The plantlets responded to exogenous PGRs as expected, since all bryophytes synthesise essential phytohormones and possess receptors for them (Sabovljević et al. 2014b). When applied individually at low concentrations, both IBA and BAP led to normal gametophore development, albeit smaller in size than in the control plants (Fig. 4A and Fig. 5B, 5C). In certain species, BAP at low concentrations can promote the formation of new shoots, as in Pterygoneurum sibiricum Otnyukova (Jadranin et al. 2023) or A. undulatum (Bijelović et al. 2004). In contrast, when BAP is applied in high concentrations, it can induce the development of abnormal gametophores with small numbers of buds and shoots. This has been documented for B. argenteum (Bijelović et al. 2004), H. heimii (Ćosić et al. 2022), and P. sibiricum (Jadranin et al. 2023). In general, cytokinins influence bud formation at the caulonemal threads, dictating the formation of new gametophores in the protonemal patches (Ashton et al. 1979). Therefore, when applied at optimal concentrations, BAP may be effective in the vegetative propagation of some moss species.
However, when applied in conventional concentrations, BAP was not favourable for the propagation of E. pulchellus, as the plants spontaneously formed a large number of new, larger shoots in the absence of exogenous cytokinins (Fig. 5A). As in the BAP treatment, the plants developed normally when grown on the medium supplemented with 0.1 μM IBA, but failed to develop measurable secondary protonemata and a sufficient number of new shoots (Fig. 4B and Fig. 5B). It appears that prolonged treatment with auxins (Bopp 1963, Cvetić et al. 2007) or high concentrations of auxins (Ashton and Cove 1990) have negative effects on the development of protonemal patches in vitro. In addition, some forms of synthetic auxins may have positive effects on morphogenesis, while others have negative ones (Sarla and Chopra 1987). Therefore, more experiments should be conducted to identify the optimal concentrations of PGRs for the micropropagation of certain species.
On the other hand, when a combination of IBA and BAP was added to the medium, this had a negative effect on the development of new shoots and secondary protonemata of E. pulchellus (Fig. 4A and Fig. 5D-5F). The plants mostly formed caulonemal cells or rhizoid-like structures, but no new buds. Generally, combinations of auxins and cytokinins are essential for proper plant development, but the optimal concentrations are difficult to achieve because responses to exogenous PGRs are often species-specific. The negative effects of the combination of IBA and BAP are well documented in the literature for the index of multiplication or secondary protonema diameter or both (e.g. Bijelović et al. 2004, Vujičić et al. 2011, Ćosić et al. 2022, Jadranin et al. 2023). In addition to the negative effects on protonemal development, IBA in combination with BAP may also lead to the formation of rhizoids in vitro (Sakakibara et al. 2003), which was also the case in this study. Interestingly, the plants grown on the medium supplemented with higher concentrations of IBA (0.3 μM) and a constant concentration of BAP (0.1 μM) developed protonemata and shoots, albeit to a much lesser extent than the plants in the control group. These results indicate that E. pulchellus regenerates better spontaneously and reproduces vegetatively without exogenously added PGRs.
Conversely, in some species cultivated under axenic conditions, the combination of auxin and cytokinins were found to stimulate the formation of new shoots, as in M. hornschuchiana (Vujičić et al. 2012), while only a low concentration of both hormones positively influenced the spreading of secondary protonemata. The moss Drummondia stricta (Mitt.) Müll. Hal. also developed better with the addition of auxins and cytokinins (Singh et al. 2017) than control plants grown on a KNOP medium. A positive effect on secondary protonema formation was also documented for Bruchia vogesiaca Nestl. ex Schwägr. (Sabovljević et al. 2012c) when IBA and BAP were applied in combination. Therefore, a possible solution for the negative effects of exogenous PGRs in E. pulchellus micropropagation could be to use other types of synthetic auxins or cytokinins or to vary the applied concentration range. It has already been suggested that IBA should be applied over a shorter period of time and at a lower concentration to achieve a positive effect in combination with BAP and avoid the negative results related to novel gametophore formations.
Acknowledgements
The authors have contributed to the special issue of Acta Botanica Croatica on the occasion of its 100th anniversary issue.
Mr. Ilija Djurić is thanked for his help during the experimental test. The study is supported by the Serbian Ministry of Science, Technological Development and Innovations, contract # 451-03-65/2024-03/200178 and 451-03-66/2024-03/200178. The work of M. Ignatov and E. Ignatova was conducted within the framework of institutional project # 121032500090-7.
