1. Introduction
Food is the basic necessity of life and amongst it, milk plays a vital role from the birth until death of a human being. It is the only food that is considered as an almost complete food on the earth and hence, the sole source of nutrition for new borns (Adeniji and Eyinla, 2019). According to a report issued by OECD-FAO world milk production is about to reach 997 million tons by 2029 (Milk – Worldwide, Statista Market Forecast, 2024)
. India is the largest milk-producing country and India's milk production rose by 4% to 230.58 M Tin 2022-23, of these, liquid milk consumption in India accounts for 50% (Ganguly et al., 2017). Being an animal-origin product of high nutritional value, it comes under the category of perishable food that often undergoes microbial or chemical spoilage (Singh, 2018). In addition, the emerging pathogen causes several diseases across the world that increase the economic burden of industry and government.
The dairy industry has devised various means and technologies to meet consumer requirements and provide safe, nutritious, and good-quality milk products (Neoκleous et al., 2022). Thus, the preservation of the nutritional quality of milk paves the way for converting milk into various milk-based products such as cheese, yoghurt, butter, paneer, ice cream, etc., through processing i.e. coagulation, and heat treatment. Shelf-life of milk and milk products has been the most important criterionfor the sustainable growth of the dairy industryin the current era. Therefore, the thermal processing techniques have evolved into higher capacity milk processing lines and equipment with faster production ratesand energy-efficient processes. The various batch operations are being replaced with continuous operations, e.g. OSTA for online milk standardization and pasteurization, TetraPak and Elecstar line with pet bottling equipment for UHT processing, CONTIMAB for butter and ghee manufacturing, TVR and MVR equipped powder plants with auto-pilot modes, and they have reduced thechances of contamination. However, due to the dark side of certain technologies regarding nutritional and physico-chemical aspectsof the product, non-thermal technologies came into the picture. The shelflife of dairy products is majorly affected by three aspects; processing, additives, and packaging. Emerging dairy processing technologies, biological additives, and various packaging systems (Fig. 1) are therefore mainly emphasized in this review article.
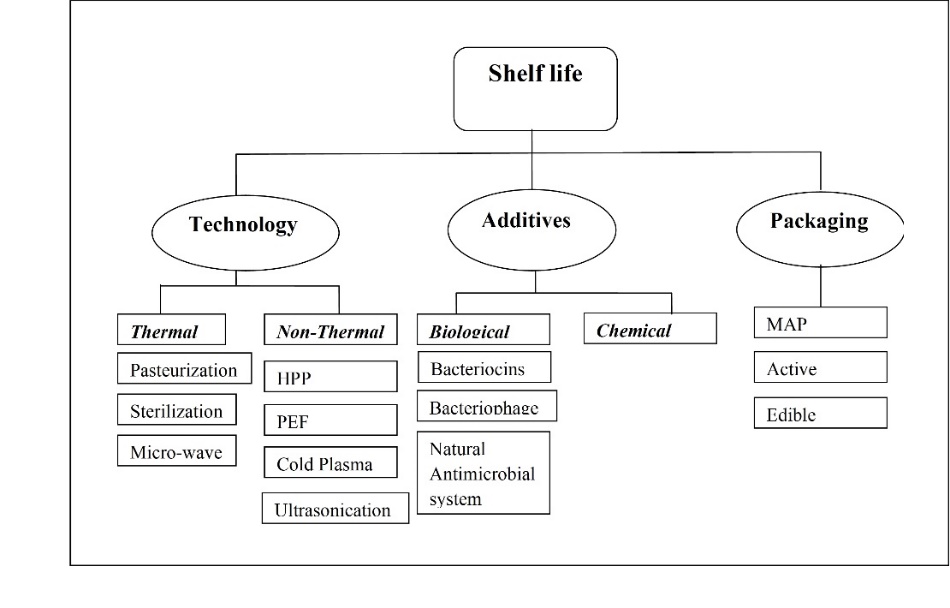
Figure 1. Classification of shelf life enhancement component
2. Thermal technologies for processing milk and milk products: pasteurization, sterilization, and microwave
Preservation of milk can be achieved by various conventional or thermal treatments like pasteurization, sterilization, freezing, chilling, drying, and the addition of chemical preservatives (Amit et al., 2017). Chilling helps in delaying the growth of bacteria by slowing down the metabolic activity whereas drying inhibits bacteria growth by lowering the water activity (aw). Both pasteurization and sterilization are well-adopted, proven, and practised industrial processes to make milk safe for consumption and to increase shelf life. Pasteurization makes milk free from pathogens and a few spoilage-causing microorganisms whereas sterilization destroys all vegetative cells. The time-temperature employed for pasteurization is 63 °C /30 min or 72 °C/15 sec and for sterilization 115-120 °C/15-20 min or >1350C/1-3 sec (Ultra High Temperature), respectively (Bezie, 2019).
Another novel version of heat treatment came in the form of microwave (MW). MWs are the electromagnetic waves between the frequency bands of 300 MHz to 300 GHz. The typical characteristic of heat generation within the material is the magnificence of a treatment. Exposure to microwave causes rapid heat generation leading to volumetric heating and a quick increase in temperature. This offers a greater advantage reducing adverse effects on nutrients over the conventional methods. The usual frequency bands; 2450+50 MHz(domestic) and 915+25 MHz(commercial),have penetration depths of 3 to 8 cm and 8 to 22 cm (Martins et al., 2019). The effectiveness of MW is significantly driven by the dielectric properties of the material. Two mechanisms are proposed for MW heating; 1) Ionic conduction; which results from resistance of food molecules to the flow of the ions and hence collisions between molecules, and 2) Dipolar rotation; associated with alternating motion of polar molecules trying to align with the alternating electric field. The effectiveness of the treatment depends on the frequency of waves applied to food, dielectric property, size, shape, and density of food.
Exposure of burfi (milk-based traditional Indian sweet)at a power level of 40% (i.e. 400w) for 7 sec showed acceptability up to 19 days at 30±1 °C and 28 days at 5±1 °C (Kumar et al., 2018), whereas, paneer (a heat and acid coagulated milk product similar to cottage cheese) treatment at a power level of 60% (i.e. 600w) for 32s was found acceptable for 8 d and 15 d at 30 °C and 5 °C (Kumar et al., 2017), respectively. Rasooly et al., 2014 added Shiga toxin Type-2 (Stx2) at a conc. of 0.5 mg/ml to milk and exposed it to two different microwave treatments; (1) 25 min, 10% power duty cycle (cycle period of 30 s) for a total of 165 kJ energy and 65 °C at the end of this cycle, (2) 20 min, 20% duty cycle for 198 kJ and 78 °C at the end of this cycle. They found a Stx2 reducing effect in milkin both methods, i.e. either with conventional heating of milk at 95 °C for 5 min or by increased microwave energy of 198 kJ. MW treatment of milk at 750 W for 75 s reduced the viable count of total mesophiles and enterococci by one log cycle whereas pseudomonas, total coliforms, and Enterobacteriaceae viable counts were decreased by two log cycles after the treatment of milk. The faecal coliforms, yeast, and lactic acid bacteria were found less susceptible to the treatment. The treatment at 900 W for 75 s gave an effect equivalent to boiling (Tremonte et al., 2014). C. sakazakiiwas inoculated (108 cfu/ml) in reconstituted infant formula followed by its MW treatment in the range of 400-900 W for 0-120 s. After treatment, the samples were stored at 4 °C for 24 h to confirm the recovery of cells. MW at 800 and 900W decreased the C. sakazakii below detectable levels, reaching maximum temperatures of 78.8±2.3 °C and 88.1±1.5 °C, respectively, and no cell recovery was observed during post-treatment storage (Pina-Pérez et al., 2014). Other similar studies are shown in Table 1.
3. Non-thermal technologies for processing milk and milk products
These processes diminish the nutritional and sensorial profile of milk and milk products. On the other hand, the increased awareness and consumer demands for fresh, minimally processed food, with good nutritional and organoleptic quality, and extended shelf life has forced the food industry to search for the new independent technologies or those combined with traditional ones (Coutinho et al., 2019). This has resulted in an innovative approach of the dairy industry towards non-thermal technologies such as High-Pressure Processing (HPP), Pulsed Electric Field (PEF), Cold Plasma, Irradiation, Ultrasonication, Ohmic heating, Microfiltration, Bactofugation, etc. to enhance the shelf life of products (Martins et al., 2019; Nikmaram and Keener, 2022). All these processes facilitate their operation at ambient temperature or a temperature less than when conventional heat treatments are used, thereby, retaining the natural content of the milk and milk products. Furthermore, microfiltration helps in removing bacterial cells as well as somatic cells through filters having a pore size of 0.1-10 µm (
Tomasula and Bonnaillie, 2015).
Table 1. Application of microwave for enhancing the shelf life of milk and milk products
|
Sr. No.
|
Type of Food
|
Treatment
|
Result
|
Reference
|
|---|
| 1 | Burfi | Burfi was treated at 400 W/7 s and samples were stored at 30±1 °C and 5±1 °C. | Burfi was acceptable up to 19 days at 30±1 °C and 28 days at 5±1 °C. | Kumar et al., 2018 |
| 2 | Burfi | Curry leaf (0.05 ppm) and clove bud (0.15 ppm) added to burfi were kept for MW heating at 0-100% power levels for 10-90 s followed by storage 30±1 °C and 5±1 °C. | The time-power level combination of 10%/60 s was found best. The treated samples were acceptable on the 8th and 12th day at 30±1 °C and 5±1 °C compared to the control, respectively.
| Badola et al., 2017 |
| 3 | Paneer | Paneer samples were treated at a power level of 60 (i.e. 600w) for 32s. Samples were stored at 4 °C and 30 °C.
| The shelf life found for paneer was 8 d and 15 d at 30 °C and 4 °C, respectively. |
Kumar et al., 2017
|
| 5 | Milk | Shiga toxin (Stx2) at a conc. of 0.5 mg/ml added to milk was exposed to two different microwave treatments; 1) for 25 min, 10% power duty cycle (cycle period of 30 s) for a total of 165 kJ energy and 65 °C at the end of this cycle, (2) for 20 min, 20% duty cycle for 198 kJ and 78 °C at the end of this cycle. | Conventional heating of milk at 95 °C for 5 min or at increased microwave energy of 198 kJ reduced the Stx2 activity. | Rasooly et al., 2014 |
| 6 | Reconstituted infant formula | C. sakazakii inoculated (108 cfu/ml) RIF followed MW treatment in the range of 400-900 W for 0-120 s. After treatment, samples were stored at 4 °C/24 h to confirm the recovery of the cell.
| MW at 800 and 900W decreased the C. sakazakii below detectable levels, reaching maximum temperatures of 78.8±2.3 °C and 88.1±1.5 °C, respectively. Post-treatment storage confirmed no recovery of cells.
| Pina-Pérezet al., 2014 |
| 7 | Milk | Effect of MWH (900 W) was compared to conventional heating methods under identical conditions of final temperature and treatment time. The initial total viable cell count was 50,000-3,50,000 cfu/cm3.
| They could not find major differences between MW heating and traditional heating. | Géczi et al., 2013 |
It can be applied in combination with pasteurization for extended shelf-life of products. Bactofugation removes bacterial spores by applying centrifugal force followed by pasteurization. The emerging non-thermal technologies are discussed herewith and relevant studies conducted to increase the shelf life of milk and various milk products are summarized in Table 2.
Table 2. Non-thermal technologies used for preservation of milk and milk products
|
Sr. No.
|
Type of Food
|
Treatment
|
Result
|
Reference
|
|---|
|
High-pressureprocessing (HPP)
|
| 1 | Milk | Milk was inoculated with 5 different strains of E. coli, Salmonella, and L. monocytogenes treated at 400, 500, and 600 MPa with a hold time at pressure of 1, 3 and 5 min at room temp. After processing, raw, pasteurized and HPP milk was stored in one-litre bottles at 4 ± 0.5 °C for ta28-day shelf life study.
| HPP (600 MPa for 3 min) significantly reduced pathogenic count, total viable counts, Enterobacteriaceae, LAB, and Pseudomonas spp. in milk, thus prolonging the microbiological shelf life of milk by 1 week compared to pasteurized milk.
| Stratakos et al., 2019 |
| 2 | UF-cheese | UF-cheeses were manufactured with recombinant chymosin (RC) or bovine rennet (BR), processed at 600 MPa/ 5 min/ 25 °C, and stored at 7 °C for up to 56 days. | Both UF-cheeses (made with RC or BR) showed a significant increase in syneresis over time. Regarding texture, HPP yielded firmer cheeses. After 1 day of storage, HP-processed cheeses made with RC and BR had a reduction of 42.8 and 52.5% in proteolysis, respectively, in comparison with the controls. Moreover, HPP promoted a reduction in the psychrotrophic count. | Ribeiro et al., 2018 |
| 3 | Milk | Two pathogenic strains – L. monocytogenes ATCC 7644 (LM) and E. coli ATCC 25922 (EC) inoculated UHT milk weretreated at 400, 500, 550, and 600 MPa/ 15 min/ with inlet temperatures 20 °C, and then stored at 4 ± 2 °C for 10 d.
| After 10 days of storage, 2 cfu/ mL of E.coli ATCC 25922 and L. monocytogenes ATCC 7644 were detected in analysed samples with lower ability to repair was detected in milk samples treated with 600 MPa/ 15 min/ with inlet temperature 20 °C)
|
Liepa et al., 2018
|
| 4 | Whey lime beverage | The packaged samples of whey-lime beverage were processed at 500 MPa/10 min/ 25 °C and stored at 40C for 120 d. A control sample was given the treatment of 90 °C for 60 seconds.
| HPP processing preserved the higher antioxidant capacity (54.2%), colour, content of phenolics (60.2%), and low non-enzymatic browning index (0.181 ± 0.03) compared to the control. | Bansal et al., 2019 |
| 5 | Mozzarella cheese | Two weeks after manufacture, 2 groups of cheese samples were treated with HHP at 500 or 600 MPa for 3 min and then returned to storage at approx. 4°C. | The results indicated that 600-MPa treatment helped minimize typical age-related changes in the performance of the reduced-Na LMPS Mozzarella cheeses. The study demonstrated that the shelf life of LMPS Mozzarella cheeses can be extended from the traditional 4 to 6 wk to 20 wk of refrigerated storage by the application of HHP at 600 MPa for 3 min. |
Ozturk et al., 2018
|
| 6 | Milk | Milk was given HHP treatment at 400 - 600 MPa/ 15 min/room temperature. | The firmness of the curd was higher than that of raw or pasteurized milk. The RCT of milk treated at 400 MPa was at par with raw milk. |
Liepa et al., 2017
|
| 7 | Bovine colostrum | Milk inoculated with E. coli, Salmonella Dublin, or MAP, bovine herpes virus type 1, and feline calicivirus were pressure processed at 300 MPa. (30, 45, and 60 min) and 400 MPa (10, 15, and 20 min).
| High-pressure processing of colostrum is effective in the reduction of native aerobic bacteria, E. coli, Salmonella Dublin, and both enveloped and nonenveloped viruses, but MAP was resistant to the effects of HPP. Calves fed with pressure-processed colostrum had similar serum IgG but lower efficiency of absorption than calves fed with heat-treated colostrum.
|
Foster et al., 2016
|
| 8 | Milk | The effect of HPP (200, 400, and 600 MPa for up to 40 min at 70 °C) on B. cereus spores was investigated. Thermal treatment was given at 70, 80, and 90 °C.
| The 600 MPa combined with heat enhanced the spore inactivation in milk, requiring a temperature lower than 20 °C to achieve the same spore inactivation. | Evelyn and Silva, 2015 |
|
Pulsedelectric field (PEF)
|
| 1 | Milk | The milk received pre-heat treatment of 40 °C and 50 °C followed by PEF of 10KV/cm, 20KV/cm, and 30KV/cm at two different treatment times (3 and 6 minutes). The samples were stored at refrigeration temperature. | The different treatment of samples showed shelf life between 16.33 ± 0.21 to 30.83 ± 0.17 (mean value). | Indumathi et al., 2018 |
| 2 | Milk | Electric field strengths of 18-28 kV/cm were applied for 17-235 µs to milk at different temperatures (4-55 °C) for 24 s. | Controlled pre-heating and PEF treatment (23 kV/cm, 17-101 µs) with intermediate cooling during PEF has the potential to provide the equivalence to thermal pasteurization conditions. | Sharma et al., 2014a |
| 3 | Milk | E. coli 916 (ATCC 25922) and L. innocua 3024 (ATCC 33090) inoculated milk was pre-heated at 55 °C for 24 s followed by PEF in a continuous mode at electric field intensities of 15.9-26.2 kV/c. for 17-101 µs.
| Combined treatment (at 55 °C) for 24 s and 15.9-26.1 kV/cm for 34-101 µs effectively reduced the organism'snumber below the detection limit of 2 log cfu/mL. Pre-heat treatment and PEF at 26.1 kV/cm for 34 µs, reduced the activity of plasmin, xanthine oxidase, and lipolysable fat by 12%, 32%, and 82%, respectively, compared to raw milk. | Sharma et al., 2014b |
| 4 | Milk | Milk was treated at 46.15 kV/cm / 20 to 60 °C/ 30 pulses (2 µs each). Then, the samples were stored at 4 °C and 21 °C for 35 d. | The treated samples showed minor physico-chemical changes. The samples stored at 4 °C were stable for33 d while samples stored at 21 °C were stable for up to the 5th d of storage.
| Bermúdez-Aguirreet al., 2011 |
| 5 | Milk | Milk subjected to PEF consisted of 5 pulses of 35 kV/cm and pulse width of around 2.3 µs, applied to milk at 65 °C. The samples were stored at 4 °C for 50 d. | The shelflife of milk was extended by a minimum of 24 days. | Sepulveda et al., 2009 |
|
Irradiation
|
| 1 | Cheese (Ricotta) | X-rays at intensities of 0.5, 2, and 3 kGy were used for sanitizing cheese manufactured artisanally and industrially. Then, samples were stored at 4 °C for 24 and 84 d. | The artisanally and industrially manufactured cheese showed a shelf life of 20 and 84 d, respectively, compared to the control. | Ricciardi et al., 2019 |
| 2 | Cheese (Fiordilatte) | Cheese samples containing Pseudomonass pp were exposed to surface UV-C light treatment at 0.1, 0.6, 1.2, and 6.0 kJ/ m2 for 5, 30, 60, and 300 s, respectively. The samples were then stored at 9 °C.
| A germicidal effect of about 1–2 log cycles on Pseudomonas spp. and Enterobacteriaceae was observed during storage. An 80% shelf-life extension was achieved by selecting adequate processing conditions of exposure of Fiordilatte cheese to UV-C light (6.0 kJ/ m2).
| Lacivita et al., 2016 |
| 3 | Cheese (Mozzarella) | Cheese samples were irradiated (10 MeV electron beam at doses of 0-2.5 kGy at 30 °C) and stored at 10 °C for 90 d. | The treatment has significantly (P<0.05) increased the shelf life of the product. | Huo et al., 2013 |
| 4 | Cheese | Milk inoculated with three different species of Mycobacterium (M. bovis, M. paratuberculosis, and M. tuberculosis) used to prepare cheese was exposed to gamma radiation at intensities of 0, 2, and 4 and stored at 4+1 °C for 15 d.
| Irradiation at a dose of 2 kGy was sufficient for the complete inactivation. | Badr, 2011 |
|
Cold plasma
|
| 1 | Cheese | Fresco cheese (QFC) and cheese model (CM) inoculated (
L. innocua) samples were treated with HVACP (100KV/ 60 Hz/ 5 min.) under the direct and indirect modes of exposure in dry air gas environment for 5 min.
| After direct HVACP treatment, a reduction of 3.5 and 1.6 log10 CFU/g was observed for CM and QFC. Direct plasma treatment was more effective than indirect. | Wan et al., 2019 |
| 2 | Milk | Sterilization of raw cow milk was performed by using the dielectric barrier discharge (DBD) plasma method. | 3kV/ 3 min/500 Hz frequency had completely killed the bacteria in raw milk. |
Aslan, 2016
|
| 3 | Milk | Plasma was generated in the milk (
E. coli inoculated pasteurized and raw) by a nanosecond pulse generator (18 kV, argon) at two different frequencies: i) 2.5 kHz, and ii) 4 kHz for 2 minutes. The samples were stored at 4 °C for 4 weeks.
| No viable cells were detected in milk treated at 4 kHz (inoculated with E. coli) and stored for up to 4 weeks after treatment.
| Ponraj et al., 2015 |
| 4 | Cheese | Sliced cheddar cheese was inoculated with E. coli O157:H7, L. monocytogenes, and S. typhimurium and exposed to flexible thin-layer dielectric barrier discharge (DBD) plasma.
| The level of these microorganisms on sliced cheddar cheese in response to 10-min plasma treatment was significantly decreased by 3.2, 2.1, and 5.8 Log CFU/g, respectively. | Yong et al., 2015a |
| 5 | Cheese | Cheese slices were inoculated with E. coli O157:H7, S. typhimurium, and L. monocytogenes treated with encapsulated DBD plasma produced at 250 W/ bipolar 15 kHz/1-15 min.
| The number of E. coli, S. typhimurium, and L. monocytogenes inoculated on cheese slices decreased by 2.67, 3.10, and 1.65 at 60 s, 45 s, and 7 min. Further reduction was observed during post-treatment storage. Thus, encapsulated DBD can be used to increase the post-treatment storage of cheese.
| Yong et al., 2015b |
| 6 | Milk | Milk was inoculated with E. coli, S. typhimurium, and L. monocytogenes treated with encapsulated DBD plasma produced at 250 W/ bipolar 15 kHz/5-10 min.
|
The total aerobic count was not detected in plasma treated sample whereas the samples inoculated with pathogenic strains showed a reduction of 2.64 log cfu/ml in case of 10 min.
The encapsulated DBD with less than a 10 10-minutetreatment period can be applied to milk to increase the shelf life with minimum physico-chemical changes.
| Kim et al., 2015 |
| 7 | Cheese Slices | Cheese slices were inoculated with E. coli and S. aureus treated with DBD plasma produced at 3.5 KV / 50 kHz/ 1-15 min.
| The number of E. coli inoculated on cheese slices decreased by 0.09, 0.47, 1.16, and 1.47 log cycles with helium (4 liters/min [lpm]) and 0.05, 0.87, 1.89, and 1.98 log cycles with He/O2 mixture (4 lpm/15 standard cubic centimeters per minute), after being treated with plasma for 1, 5, 10, and 15 min, respectively. Significant reductions were also observed in S. aureus inoculated onto cheese slices ranging from 0.05 to 0.45 log cycles with He and from 0.08 to 0.91 log cycles with He/O2-treated samples, respectively.
| Lee et al., 2012 |
| 8 | Milk | Low-temperature atmospheric pressure plasma treatment (9 kV AC/ 0-20 min) was given to E. coli ATCC 25922 inoculated UHT milk (whole, semi-skimmed and skimmed milk) samples followed by storage at 4–7 °C for 6 weeks.
| A significant 54% reduction in the population of E. coli cells after only 3 min was observed regardless of the fat content of the milk. The levels of E. coli for 20 min plasma applied samples were undetectable after one day of storage and remained thereafter at the end of a 6 week period.
| Gurol et al., 2012 |
|
Ultrasound
|
| 1 | Milk | Milk samples were sonicated at 2,200 W/ 20 kHz/10-60 s. Thermo-sonication was performed at 72.5+0.3 °C (mean+SD) after pasteurization (72.5+0.3 °C) (mean+SD), whereas cold-sonication was carried out at 12.5+5°C (mean+SD) before pasteurization. Then, milk was refrigerated up to 50 d. |
Counts in pasteurized controls and C-S milk
did not exceed 3.00 log cfu/mL for up to 50 d; counts in T-S milk exceeded 5.00 cfu/mL by d 36. Neither C-S nor T-S were appropriate techniques for reducing bacterial count in fluid milk beyond standard pasteurization and, in fact, increased counts of spore-forming spoilage bacteria.
|
Lim et al., 2019
|
| 2 | Milk | Pasteurized and UHT milk (inoculated) received high-intensity ultrasound treatment (range from 0 to 84 °C, amplitude range from 0 to 216 µm, and time range from 0.17 to 5 min). | Optimization of the inactivation of microbes was found to be at 84.8°C, 216 µm amplitude, and 5.8 min. |
Ganesan et al., 2015
|
| 3 | Milk | The milk samples inoculated with a cocktail of L. monocytogenes strains (two) were exposed to ultrasonic oscillations where frequencies were switched to 28, 45, and 100 kHz at 1 ms time intervals.
| The test organisms exhibited biphasic inactivation curves in all milk samples. The correctedD-value was shortest in full-cream milk at 24.81 min, followed by those in nonfat and low-fat milk at 29.17 and 30.64 min, respectively. | Gabriel et al., 2015 |
| 4 |
Queso fresco cheese
| Raw milk was sonicated at 400 W/ 24 kHz/ 120 μm and heated at two different time-temp combination; 63 °C/10-30 min or 72 °C/0.15-1 min. Samples were stored at 4 °C for 23 d.
| Curdling time was reduced considerably, cheese yield (20.6%) was almost doubled, and the luminosity of cheese was increased (
L*). Cheese processed at 63 °C/ 120 μm/30 min had the best quality. Shelf life was extended considerably and the product had higher quality.
| Bermúdez-Aguirreand Barbosa-Canovas, 2010 |
| 5 | Milk | Milk (UHT) inoculated with E. coli, L. monocytogenes, and P. fluorescens received treatment at 750 W, 20 kHz, 124 μm/ 2.5-10 min.
| Viable counts of E. coli, P. fluorescens and L. monocytogeneswere reduced by 100% after 10.0, 100% after 6.0 min, and by 99% after 10.0 min.
| Cameron et al., 2009 |
High-pressure processing (HPP)
HPP is considered as one of the most emerging techniques in the last few decades. First time Hite demonstrated the shelf life extension of raw milk by applying HPP. Later on in the 20th century, Japan successfully manufactured and marketed HPP-treated fruit and jams. It can inactivate the spoilage of foods by delaying the onset of enzymatic and chemical deteriorative processes. It works on two principles; 1) Le Chatelier’s: pressure favours all structural reactions and changes that involve a decrease in volume and 2) Isostatic principle: the distribution of pressure is proportional in all parts of a foodstuff irrespective of their shape and size. The food is exposed toa high pressurein the range of 100-1000 Mpa.(
Voigt et al.,2015). Water or a mixture of oil or alcohol can be used as a pressure-transmitting medium. The temperature is increased at a tune of 2-3 °C per 100 MPa. The treatment below 420 MPa produces an effect similar to pasteurization and above 700 MPa similar to sterilization. Inactivation of spores required much higher pressure and temperature than vegetative cells. In Figure 2, general structures/organelles present in a bacterial cell are depicted. In the majority of thermal and non-thermal methods, the cell wall components and cell membrane of bacteria usually get affected. Specifically, the proposed mechanism of microbial inactivation is related to the loss of cell membrane permeability (Fig. 3) and the breaking down of cell membranes, cell walls, etc.
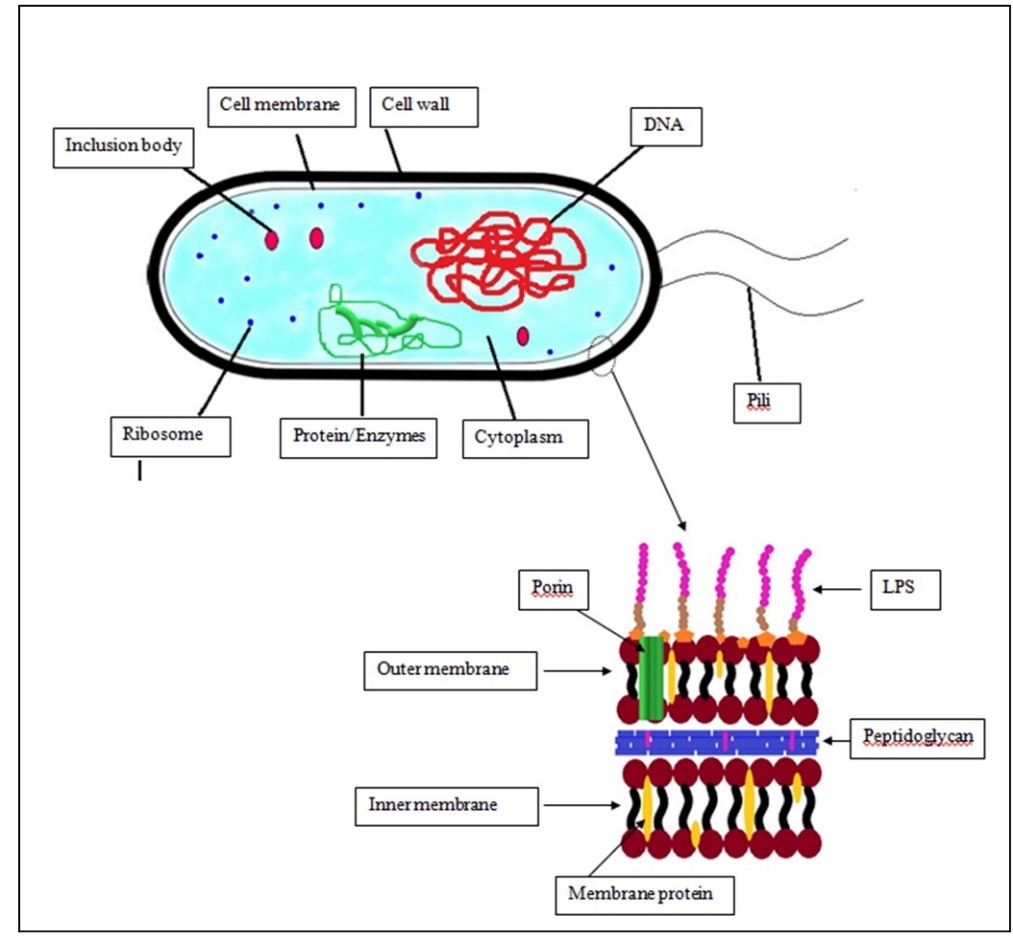
Figure 2. Bacterial cell structures and sectioning of cellwall of Gram positive bacteria
Pulsed electric field (PEF)
PEF has been successfully applied to alter the genomic material amongst microorganisms by inducing electroporation (perforating cell membrane). In the 19th century, it was used toinactivate the enzymes and MOs in food products. The food is placed between two electrodes and short pulses (1-10 µs) are generated through a high voltage (20-80 kV) pulse generator. Pasteurization of milk is generally performed with square-wave. A number of functional and structural changes takeplace in cell membranes due to high-voltage treatment-led microbial inactivation. Critical transmembrane potential or Critical electric field (
Ec) (kV/cm) indicates the maximum potential difference that the cell membrane can withstand. A higher external electric field than Ec forms irreversible pores whereas a lower external electric field than Ec forms reversible pores. The pore formation leads to cell death (Fig. 4). Both, static and continuous systems are used to process food (
Sampedro and Rodrigo, 2015). Example of companies manufacturing PEF processing units are Diversified Technologies Inc. and PurePulse Technologies Inc. in the USA and ScandiNova Systems AB in Sweden with overall flow rates ranging from 400 to 6000 l/h. The process is affected by various factors such as process time, voltage applied, physiological state of cells (log phase is more susceptible), conductivity, and pH of the product.
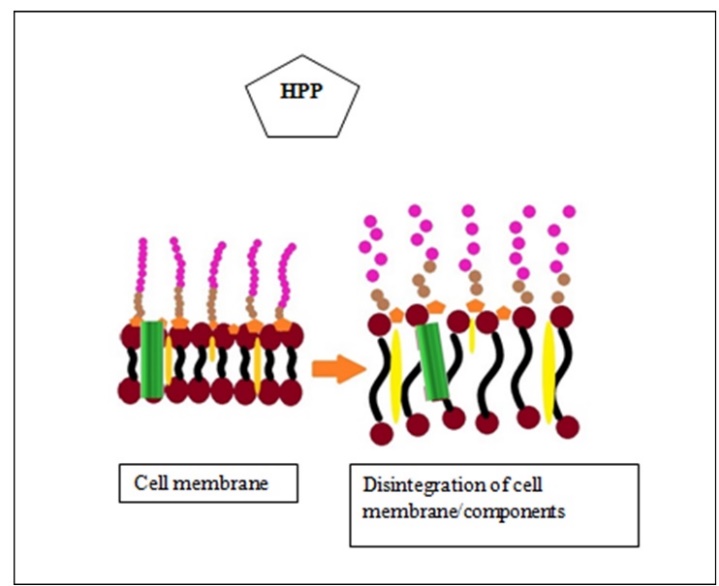
Figure 3. Cell membrane damage by high-pressure processing (HPP) (modified from Naik et al., 2013)
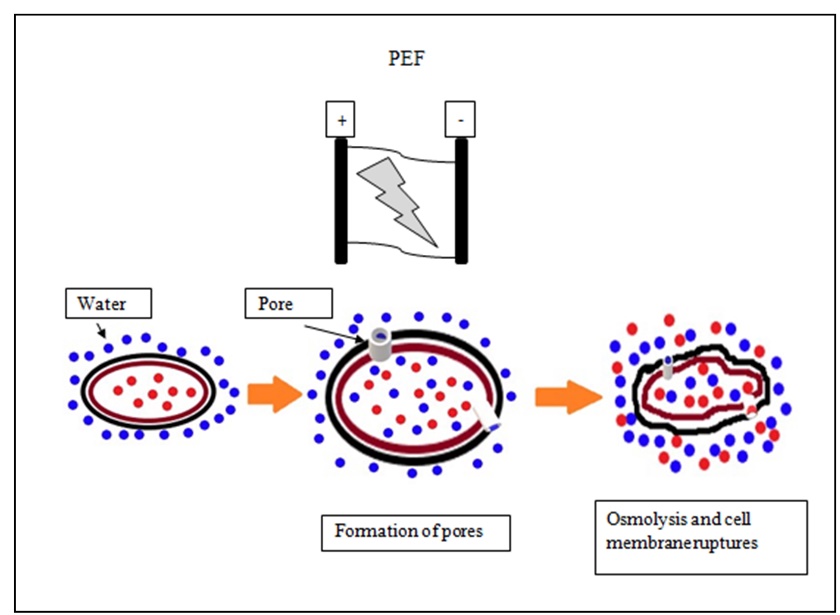
Figure 4. The microbial cell destruction by Pulsed Electric Fields (PEF) (modified from Sampedro and Rodrigo, 2015; Sharma et al., 2014 a, b)
Cold plasma
Plasma is a Greek word meaning mouldable substances, first explained by Irving Langmuir in 1920s (Baghya and Narayanan, 2019). It is referred to as the fourth state of matter, electrically neutral, and produced in the presence of energy sources (i.e. electricity) and gas. It is found electrically neutral. Based on the method of generation, pressure, and relative temperature, plasma is classified into two groups; a) Thermal- produced under high pressure and power where gas species and electrons are in thermodynamical equilibrium in nature, and b) Cold or Non-thermal - produced under reduced pressure (at atmospheric) and power where gas species and electrons are thermodynamically non-equilibrium in nature. Cold plasma is found suitable for the application in food industry because the ions or uncharged molecules gain only little energy and remain at a low temperature. The products of plasma includeN2, NO, NO2, nitric oxide radical NO+, atomic oxygen (O), ozone (O3), ions, neutrons, protons and reactive oxygen and nitrogen species, and hydroxyl radicals (OH+) (Coutinho et al., 2019; 2021; Nikmaram and Keener, 2022).
Cold plasma is generated by various methods such as Dielectric barrier discharge (DBD) method, atmospheric plasma jet discharge, corona discharge, and microwave-driven discharge. MW-driven discharge uses microwaves instead of electrical fields. Air or nitrogen or a mixture of noble gases like argon, neon, and helium are used in the presence of the electric field. The most commonly used method in dairy industry is DBD. It has been successfully applied to destroy pathogenic as well as spoilage-causing organisms in cheese, raw milk, cheese slices, etc. The effectiveness is significantly affected by Relative Humidity (RH), type of species generated, length of treatment, and power level used for generation. The RH is directly correlated with the reactive species generated, e.g. the higher the RH, the more the peroxyl acid groups and OH there will be. The radical bombardment causes several phenomena on/in a microbial cell, i.e. formation of lesions, mutational damage to DNA and/or RNA by breakage of chemical bonds, lipid oxidation or peroxidation in the cell membrane, denaturation of cell protein or enzymes as depicted in Fig. 5 (Coutinho et al., 2019). Therefore, one or more of such effects led to cell injury or viability. It is an energy efficient method and is applied on both solid and liquid food at low temperatures. However, the lipid oxidation and the low penetration power are the limitations for the application of cold plasma in dairy products since dairy products are rich in triglycerides-natural milk fat together with a colloidal form of protein (casein) that protect the microbial cells against the potential damage caused by cold plasma method.
Ultrasonication
The method refers to the application of sound waves at a frequency (>16 kHz) greater than the upper limit of human hearing through liquid, solid, or gases which causes vibration, acoustic streaming, and formation of small bubbles (known as cavitation) due to pressure variation. Two types of cavitations occur; a) transient (20 kHz) and b) stable (>200 kHz). The size of the bubble increases and when it attains a volume at which itcan no longer absorb energy, it implodes violently at higher-intensity waves. This generates mechanical, physical, and chemical effects, such as shockwave formation and turbulent motion. Both, the temperature and pressure are high during implosion (Ashokkumar et al., 2010). The treatment involves direct (using ultrasonic probe) and indirect mode (ultrasonic bath). It has been found to be an important tool for various applications, i.e. drying, crystallization, extraction, filtration, emulsification, cleaning, etc.
The combination of heat and ultrasonication is more lethal (known as thermosonication). Another approach includes pressure+ultrasonication (Manosonication) and heat+pressure+sonication (Manothermosonication) (
Zisu and Chandrapala, 2015). High-intensity ultrasonication tends to develop undesirable off-flavour. On the other hand, mild-level ultrasonication helps in improving quality, reducing fermentation time and enzyme productivity. Cell inactivation occurs by disrupting both cell wall structure and function through cavitation in an ultrasonicator.
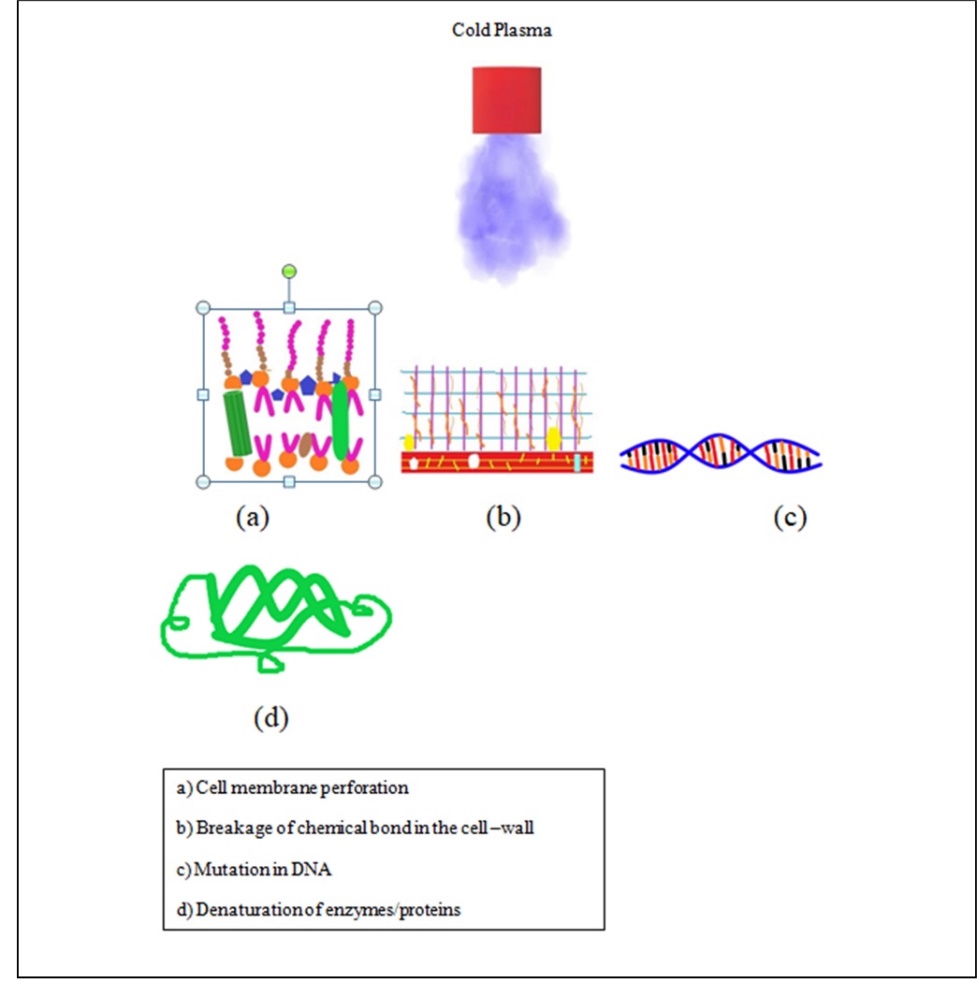
Figure 5. Various modes of bacterial inactivation by cold plasma (modified from Coutinho et al., 2019)
Irradiation
The substantial reduction of microorganisms at ambient temperature without affecting the vitamins, flavour, and colour makes this technique appropriate for the processing of milk and milk products. Food is exposed to ionizing radiation in the form of Gamma (Cesium 137 or cobalt 60), X-rays, ultraviolet light and electron beams. Radiation dose is measured by a Dosimeter device and expressed in terms of Gray (Gy). WHO has endorsed the use of irradiation doses up to 10 kGy in foods. UV light can potentially reduce the microbial count without affecting other properties of food (Roberts, 2016). Based on wavelength, UV rays are classified as UV-A (315 and 400 nm), UV-B (280–315nm), and UV-C (200-280 nm)(
Datta et al., 2015). UV-C is most effective in food processing.
The composition and transparency of food affect the efficacy of UV light. As the milk is opaque in nature due to colloidal and suspended solids, two approaches have been suggested to obtain complete penetration; a) turbulent flow of milk exposes all surfaces to UV light and also reduces the path length, and b) laminar flow that forms thin layer of milk on UV irradiated surfaces. It tends to mutate the bacterial cell by forming pyrimidine dimmers (Fig. 6) that block DNA transcription and replication causingbacterial cell death. The irradiation dose of bacterial inactivation is much lower than required for algae, viruses, and fungi. The radiation is successfully applied for food application on a commercial scale in over 26 countries. The irradiated products are being identified through the “
Radura” logo labeled on packed food. Irradiation of cheese significantly increases the shelf life along with the inactivation of microbes (Badr, 2011;Ricciardi et al., 2019).
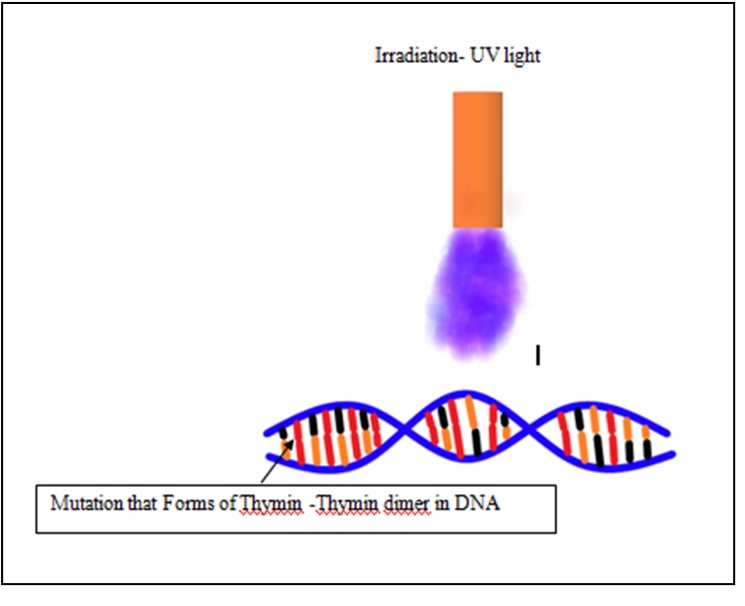
Figure 6. DNA damage by irradiation (adapted and modified from Huang and Zhou, 2020)
Ohmic heating
In the 19th century, James Joule, hence named as Joule heating, revealed the heat transfer upon passage of electric current passed through food. Electrical resistance is found responsible for heat produced inside the food and the unit of measurement of resistance is Ohm (Ω), therefore, also referred to as Ohmic heating (OH). In the year 1919, Anderson and Finkelstein were first to recommend OH for milk. The initial successful commercial technique was known as “Electro Pure”. The movement of ions in the liquid causes collision, which in turn, results in creating resistance and generation of heat (Sakr and Liu, 2014; Kumar, 2018). Factors like electrode type, conductivity, concentration of ions, field strength, etc. greatly influence the effectiveness of OH in food. OH of milk products improves the texture as well as the shelf life of products (Sun et al., 2008; Kumar et al., 2014; Parmar et al., 2018).
Similar to this, Light-emitting diodes (LEDs) are another eco-friendly light source with wide-spectrum antimicrobial activity against bacteria, fungi, viruses, planktonic cells, and endospores (Yu et al., 2022).
4. Bio-preservation
Bio-preservation makes the use of metabolites/substances that are produced by microorganisms or entire cells or present naturally in milk. Bacteriocins, lysozyme, lactoferrin, natamycin, bacteriophage, and endolysins are the different kinds of biopreservatives, that obtained the designation Generally Recognized as Safe (GRAS), and can be employed to enhance the shelf life of milk and milk products (Table 3) (Conte et al., 2011). Nisin and pediocin are well known examples of bacteriocins, which are proteinaceous compounds, produced by LAB to inhibit the growth of similar or closely related bacterial strain(s).These compounds,either alone or in combination with other antimicrobial agents, (like sorbic acid, EDTA) have been shown to inhibit the growth of spoilage and pathogenic bacteria (
E.coli, Bacillus sp., L. monocytogenes, Staph. aureus, etc.) in several milk products(Conte et al., 2011; Chawla et al., 2015). Natamycin (E235) is an antifungal compound produced as a secondary metabolite by some species of Streptomyces; it is effective at very low levels (MIC is less than 10 ppm) for most moulds. It has been successfully used to preserve various types of cheeses, sour cream, yoghurt, and packaged salad mixes(Saad et al., 2015).
Table 3 Use of bio-preservatives and essential oils and herbs for milk and milk products preservation
|
Sr. No.
|
Type of Food
|
Treatment
|
Result
|
Reference
|
|---|
|
Bio-preservative agents
|
| 1 | Pasteurized milk | Milk was inoculated with MRSA CCARM 3089 cells (2 x 105 CFU/mL) and LysSA11 at 0, 1.125, 2.25, 3.375, 4.5, and 9 μM. The milksample was then incubated at 4 °C or 25 °C for an additional hour. | In milk stored at 4 °C and 25°C, significant inhibitory effects (
P<0.05) were shown within 15 min by 3.375 μM of LysSA11. Moreover, viable cells were reduced to undetectable levels at 1 h (4°C) and 30 min (25°C) by treatment with 9 μM of LysSA11.
| Chang et al., 2017a |
| 2 | White mould-ripened cheese |
The treatments include, partial purified bacteriocin-like substances (PPBLS),
and bacteriocin producer isolate LAB 100.
| The concentration at 8 mg/ ml was the most suitable one to minimize the growth of P. candidum and hence extended the shelf life of Camembert cheese.
| Khider, 2017 |
| 3 | Pasteurized milk | Milk samples were inoculated with S. aureus RN4220, LysSA97, and carvacrol at 105 CFU/mL, 1.88 μM, and 6.66 mM. The samples were kept at room temperature for 3h.
| The number of live S. aureus cells decreased below the detection limit using the cocktail of LysSA97 and carvacrol conc. over 3 h.
| Chang et al., 2017b |
| 4 | Milk | L. monocytogenes and Bacillus spores contaminated milk (Skim and Whole) was supplemented with free and encapsulated commercial nisin (0.25-1.0 mg/L, alone and combined) and stored at 6±1 °C for 21 d.
| In both skimmed and whole milk, free and encapsulated Nisaplin® combined (0.5 mg/L each) exhibited the strongest antibacterialeffect, although Lm-resistant cells were observed. Free and encapsulated commercial nisin (0.25 mg/L) were highly effective against Bc spores germination and for the pathogen inhibition in both types of milk, improving the food product microbiological safety. | Martinez et al., 2016 |
| 5 | Yoghurt | Yoghurt incorporated with 25 RU/ml nisin was stored at 10-15°C for 40 d. | Application of nisin for shelf-life extension of stirred yoghurt made from cow milk is suggested. | Sarkar, 2016 |
| 6 | ESL Milk | The milk contaminated with L. monocytogenes 2000/47 treated with phage FWLLm1 added at 5 × 106 PFU/ml, FWLLm3 at 5 × 105 PFU/ml, and bacteriocin coagulin C23 at 584 AU/ml was stored at 4 °C for 10 days.
| The combination of FWLLm1 and coagulin C23 reduced the count of L. monocytogenes 2000/47 below detection limits (less than 10 CFU/ml) from day 4 until the end of the experiment.
| Rodríguez-Rubio et al., 2015 |
| 7 | Processed cheese sauces | Processed cheese sauces with different preservative systems; Nisin, Nisin + Natamycin, Nisin + Potassium sorbate, or Nisin + Natamycin + Potassium sorbate were prepared. The melted processed cheese sauce was purred into glass jars (150g) and stored at room temperature (25 ± 2 °C) for 3 months. | The combination of nisin + natamycin + potassium sorbate mixture was the most effective one for the product shelf life. |
Saad et al., 2015
|
| 8 | Pasteurized milk | Pasteurized S. aureus ATCC33591 inoculated milk supplemented with overnight cultures of L. lactis CCTCCAB20102111 and L. casei BL/pBLysdb were used to prepare cheese.
| Compared with the raw milk, the viable counts of S. aureus were reduced by 105-fold in the cheese inoculated with the engineered L. casei strain during the fermentation process, and the pathogenic bacterial numbers remained at a low level (104 CFU/g) after 6 weeks of ripening at 10 °C.
| Guo et al., 2016 |
| 9 | Doda burfi | Pediocin (0.12%), microgard- 100 (0.5%), along with chemical preservatives such as potassium sorbate (0.1%) and sodium EDTA as chelating agent (20mM) were added in combination to enhance the shelf life of the product. | The shelflife of 27 days of the treated product counterpart to 12 days of control product kept at 30 °
C was recorded.
|
Chawla et al., 2015
|
| 10 | Pasteurized milk | Nisin was added at the rate of 50, 100, 200, and 300 IU/ml at about one hour before pasteurization. After pasteurization, samples were stored at 4±1°C for 16 d. |
In the case of nisin added samples, overall
acceptability scores remained within the
acceptable limit until 16 days of storage.
|
Radha, 2014
|
| 11 | Milk pudding | Nisin A was added to milk pudding to final concentrations of 40, 80, 120, and 240 IU/ ml against spores from B. thuringiensis, B. cereus, and P. jamilae. The samples were incubated at 15, 20, and 30°C for 29 days.
|
Nisin A (at ≥ 80 IU/ g in 5.0% fat and at
≥ 120 IU/ g in 7.5% fat) was found to be effective as a natural preservative to control spoilage bacteria and extend its shelf life (4 weeks).
| Oshima et al., 2014 |
| 12 | UHT and Raw | A cocktail of E. coli phage (EC6, EC9, and EC11) was tested and added to milk samples inoculated with E. coli strains (
E. coli ATCC 25922, E. coli O5:H- and E. coli O127:H6). The samples were incubated either at 25 °C for 24 h, or in a domestic refrigerator (5-9 °C) for 168 h.
| A cocktail of the three phages completely inhibited E. coli ATCC 25922 and E. coli O127:H6 in both UHT and raw milk at 25 °C and at 5–9 °C. A cocktail containing EC6 and EC9 completely inhibited E. coli O5:H-, an enterohemorrhagic strain, in UHT milk at both temperatures, whereas in raw milk regrowth was observed.
| McLean et al., 2013 |
| 13 | Cheese (Burrata) | Lysozyme (150, 250, and 500 ppm), and 50 m
M Na2-EDTA were added to the cheese and packed under MAP. The samples were kept at 8°C for 9 d.
| The shelf life of cheese was increased specifically with the highest content of lysozyme. |
Conte et al., 2011
|
| 14 | Pasteurized milk | Pasteurized milk was inoculated with 102 cfu/ml and 105 cfu/ml of S. aureus Sa9. Immediately after, nisin (0.37 μg/ ml and 0.75 μg /ml), endolysin (7.5 U/ml and 15 U/ml), and a mixture of both, were also added. The samples were kept at 37 °C for 10 h.
| Clearance of the pathogen was only achieved by the combined activity of both antimicrobials (after 6 h of incubation). | García et al., 2010 |
| 15 | Galotyri (Greek soft-acid curd) | Nisin was added to cheese at two different concentrations (N1, 50 IU/g, N2 150 IU/g) and stored aerobically under refrigeration for a period of 42 days. | The use of nisin extended the shelf-life of fresh Galotyri cheese stored at 4 °C by ca. 7 days (N1) and 21 days (N2) with cheese maintaining good sensory characteristics. | Kykkidou et al., 2007 |
|
Essential oils and herbs
|
| 1 | Peda | Peda was incorporated with 1% black pepper and 1% turmeric and stored at (7±1 °C) for 48 d. | The shelf life of herbal pedawas 48 days at
7 °C.
|
Panday et al., 2018
|
| 2 | Fresh acid-curd soft cheese | Cheese was fortified with (2, 3, and 4 %) Moringa oleifera extract and supplemented with L. plantarumand L. mesenteroides (1:1). The samples were stored at
5 °
C ± 1 for 30 d.
| Ethanolic extract of Moringa oleifera leaves can be used to add nutritional value and to extend the shelf life of cream cheese.
| Mohamed et al., 2018 |
| 3 | Labneh | The samples were prepared from UF retentate of buffalo’s skim milk and fortified with Moringa oleifera oil using three different ratios:10, 15, and 20% and L. acidophilus, too. The samples were stored at 5 °
C ± 1 for 30 d.
| The productcan be considered a new functional product with extended shelf life. | El-Sayed et al., 2017 |
| 4 | Ghee | Ghee was treated with curry leaves (0.1%, 0.2%, 0.3%, and 0.4%) and stored at 80±1 °C for an accelerated storage stability test for 12 days. | The additionof curry leaves at the final stage of heat clarification was found more effective than at the initial stage of heat clarification. The optimum rate of curry leaves found was 0.3%. | Kapadiaand Aparnathi, 2017 |
| 5 | Cottage cheese | Five different (parsley, dill, pepper, garlic, and rosemary) herbs were incorporated in fresh and dried form at 0.5. 1.0 and 2.0%. The samples were stored at 4 °
C ± 1 for 3 d.
| The sensorial best result was obtained with fresh sweet red pepper whereas dry rosemary had the highest antioxidant and antibacterial activity. |
Josipović et al., 2015
|
| 6 | Flavouredmilk | Essential oils were added at 0.005% (eugenol and trans-cinnamaldehyde enriched) in milk and stored at 4-7 °C for 7 d. | Increased antioxidant activity of the product, ultimately enhances the shelf life of the product. |
Samaddar et al., 2015
|
| 7 | Labneh | Labneh was supplemented with essential oils cinnamon, cumin, and mint oils, to a final conc. of 0.3, 0.5, and 0.8%. The samples were stored at 6±1 °
C for 24 d.
|
It can be concluded that 0.3% of cinnamon can be used to increase the shelf life of labneh for up to 24 days, with a higher level of total volatile free fatty acid and therapeutic bacteria counts and a low level of total viable, mould and yeast count.
|
Thabet et al., 2014
|
| 8 | Paneer | Brine dipped (5%) and dry salted (3%) masala paneer (coriander leaves (1%), mint leaves (1%) and green chilies (0.3%), roasted and groundcumin seeds (0.3%) and black pepper (0.3%)) were prepared and stored at 4±1 °
C for 8 d.
| The products were microbiologically safe and remained so for at least 6 days when stored in a refrigerator. | Rani et al., 2014 |
| 9 | Clarified butterfat | Ethanolic extract of Arjuna was added at 7% in butter fat. The prepared ghee samples were stored in a hot air oven at 80±1 °C for an accelerated storage stability test and analyzed at regular intervals of 0, 2, 4, 6, 8, and 10 days. | The shelf life of the Arjuna ghee samples was 8 days at 80±1 °
C as compared to 2 days in the control.
| Parmar et al., 2013 |
| 10 | Butter | 2% of dried rosemary herb and sage was added to butter. The samples were packed and stored at 4 °C for 5 months. | The results of chemical analyses suggest that addition of rosemary herb was more effective in retardation of lipolysis than supplementation with sage. However, both supplemented products were less stable during storage than the control sample. |
Najgebauer-Lejko et al., 2009
|
| 11 | Labneh |
Three essential oils, namely thyme, marjoram, and sage, were added to concentrated yoghurt (labneh) at conc. of 0.2, 0.5, and 1.0 parts per million (ppm) and stored at 5°C ± 1 for 21 d.
|
Thyme, marjoram, or sage at 0.2 ppm can be used to increase the shelf life of labneh for up to 21 d.
|
Otaibi and Demerdash, 2008
|
Lysozyme is a glycoside hydrolase that catalyzes the hydrolysis of 1, 4-beta-linkages between N-acetyl-D-glucosamine and N-acetylmuramic acid residues in peptidoglycan- the major component of Gram-positive bacterial cell wall. Furthermore, the recent approaches deal with the application of phages infecting the bacteria known as “Bacteriophage”. Being obligate parasites, they must first infect host cellsto multiply. Bacteriophages follow either the lysogenic or lytic cycle. In addition, two types of proteins are encoded by phages, namely virion-associated peptidoglycan hydrolases (VAPGHs) and endolysin (Chang et al., 2017a;2017b). VAPGHs are associated with the initial phage infection step while endolysins mediate bacterial lysis. Because of host specificity, phages have drawn the attention of food researchers and have been accepted as green technology to safeguard food. Phages have found many other applications other than bio-control, such as improving crop yields, sanitizer for farm facilities, animal and animal handling, treatment and prophylaxis in cattle, etc. Several commercial preparations (SalmoFresh™ and PhageGuard S™, PhageGuard Listex™) havebeen approved and granted GRAS status by FDA to prevent the growth of pathogens. Several studies haveshown the successful application of host-specific bacteriophages and endolysins which are hydrolytic enzymes synthesized by bacteriophages to cleave the host's cell wall during the final stage of the lytic cycle.
Herbs and essential oils
Herbs and spices have played remarkable rolesnot just as a food flavouring substances, but also as a medicine and preservative forcenturies. Indeed, there is a great linkage between diseases and food habits. India is known asthe “Botanical Garden of the World” being the largest producer of medicinal herbs. They are valued as equivalent to gold or jewels in many countries. Around 70-80% population in developing countries and 60% world’s population rely directly on herbs and plants for their medicinal uses. Moreover, as per the report of the World Health Organization, modern or non-conventional medicines based on herbal sources occupy a significant part in primary healthcare of 70% world population(Oraon et al., 2017). According to Ayurveda, the herb/spice is used as a whole plant including the root/rhizomes (
turmeric), leaves (tulsi, phudina), bark (cinnamon), flower (clove), fruit (cardamom, ashwagandha), etc., whichspecifically contains phytosterols, antioxidants, essential oils, vitamins, and other substances that help to inhibit the growth of microorganisms(Oraon et al., 2017). On the other hand, essential oils are plant-derived aromatic oily liquors, that possess antimicrobial properties and are also known for their therapeutic properties (for instance diuretics, anti-inflammatory, antiseptic, carminative, antispasmodic, and tonic substances). EOs are produced by steam distillation and have gained the status of GRAS, too. Several studies have indicated shelf life extension of different milk products viz.kinds of milk, fermented milkand ghee using herbs and EO (Table 3).
Chemical preservatives
The incorporationof chemical agents in foodhaving antimicrobial activity has been practiced since ancient times. Benzoic acid or its sodium salt, benzoate wasthe first molecule approved in the USA. Others include salt, sugar, inorganic salts, etc. (as per the Appendix- A, FSSR, 2011). Another reason to be added todairy products may deal with the antioxidant propertiesof chemical preservatives. However, several chemical preservatives have been found to have negative and life-threateningeffectson human health, particularly infants.
5. Packaging
Both, the packaging system and the packaging material of milk and milk products act as external means of preservation after processing,during transportation and distribution(Ščetar et al., 2019; Vieira et al., 2019). It performs various functions like protection, convenience, communication, containment, etc. In the 19th century, milk was packed using glass bottles. The customer’s demand for fresh, convenient, easy-to-cook food and the industrial threat of wastage, non-recyclable packaging and global warming issues brought technological innovations in the form of Vacuum packaging, Modified Atmosphere Packaging (MAP), Active packaging, Edible coating, Intelligent or smart packaging, etc. Various packaging materials include LDPE, HDPE, LLPDE, Polystyrene, EV-OH, Aluminium foil, etc. The focus in the next section is given to various packaging systems rather than packaging material. The studies on various packaging systems to enhance the shelf life of milk and milk products are depictedin Table 4.
Table 4. Various packaging systemsused for enhancing the shelf life of milk and milk products
|
Sr. No.
|
Type of food
|
Treatment
|
Result
|
Reference
|
|---|
|
Active packaging
|
| 1 | Macaroni and cheese | Ready-to-eat macaroni and cheese filled in novel oxygen scavenger and metal oxide–coated high-barrier polymer packages, were processed in pilot scale 915-MHz microwave-assisted thermal sterilization system (MATS). Also, aluminum foil packages were processed in the Allpax retort system to compare packaging performance. The samples were stored for 6 months at 37.8 °C. | The results indicated that oxygen scavenger and high-barrier packaging can be used for ready-to-eat meals with extended shelf life for soldiers and astronauts. | Patel et al., 2019 |
| 2 | White fresh cheese | The sample was stored in PET waste-based active packaging films (containing chitosan and Ag: silver nanoparticles, at three storage temperatures (6, 25, and 40 °C), for various durations (0-30 days). | 5% Ag–Cs–PET90:10 film is efficient enough to make cheese bacteria-free within 7 days at 40 °C. The PET waste-based active packaging film is found to be capable ofa shelf life extension of white fresh cheese for up to 30 days. | Singh et al., 2018 |
| 3 | UF cheese | L. monocytogenes inoculated cheese samples were put in polyethylene bags (cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin (500 ppm and 1000 ppm)), and stored at 4 ± 1 °C for 14 days.
| Films with 1000 µg/mL of nisin completely inactivated L. monocytogenes cheese after storage at 4 °C for 14 days.
| Divsalar et al., 2018 |
| 4 | Mozzarella cheese |
Cheese slices were inoculated with 106 spores/ml of Alatoxigenic strains of P. digitatum CECT 2954 and A. parasiticus CECT 2681 packed with either of the following antimicrobial devices: (1) paper filter with AIT (; (2) AIT sticker; or (3) oriental mustard meal pouchand were placed in plastic bags or plastic trays composed of MAP material. The samples were kept at 4°C and were observed for 60 d.
AIT= Allyl isothiocyanate
| Doses ≥4 μL/L of AIT may significantly increase the shelf life and safety of sliced mozzarella cheese. |
Tracz et al., 2018
|
| 5 | Butter container | Two types of oxygen absorbers adhesive labels and sachets and two caps with and without adjustable closure were tested. | The best results were achieved with the oxygen absorber sachets and using caps with adjustable closure. Under these conditions, the oxygen concentration inside the container remained below 3% during 150 h. | Otero-Pazos et al., 2018 |
| 6 | Cheese | Two active films based on chitosan (1.5% w/v) and methylcellulose (3% w/v) enriched with natamycin (0.01% (w/v)) were used to cover and store cheese at 20 °C for 7 days. | A significant reduction in yeast and mould was observed in cheese samples treated with chitosan films containing natamycin (p < 0.05). |
Santonicola et al., 2017
|
| 7 | Q
ueso Blancocheese
| Cheese was inoculated with a cocktail of the L. monocytogenes strains wrapped in the foxtail millet starch (3.5%, w/v) and clove leaf oil (1.0%), and stored at 4 °C for 24 d.
| The FMS films that contain clove leaf oil can be used as a new packaging material for enhancing the shelf life of Queso blanco cheese.
|
Yang et al. 2018
|
| 8 | Milk | Milk (2% Fat, 131.1 °C for 2 s) was stored in HDPE packages consisting of TiO2 at 3 levels (low: 0.6%; medium: 1.3%; high: 4.3%) at 3°C for up to 43 d. Light-protected (translucent, foil-wrapped) and light-exposed (translucent) HDPE packages served as controls.
| The high TiO2-HDPE package provided protection similar to the light-protected control package through 22 days of light exposure, with less consistent performance thanthe medium TiO2 package.
|
Johnson et al., 2015
|
| 9 | Milk pomade sweet-Sherbet | The samples were packed in different packaging materials using several packaging technologies (MAP). For reduced oxygen packaging (ROP) creation (O2 – 0%) in pouches, an iron-based oxygen scavenger sachets of 100 cc were used. The samples were stored at the room temperature of +21±1 °C for 12 weeks.
| Met. BOPET/PE and Aluthen are considered the best material for extending the shelf-life. | Ungure et al. 2012 |
| 10 | Fior di Latte cheese | Three concentrations (10, 15, and 20 mg) of silver montmorillonite embedded in agar were used to pack the cheese during storage at 10 °C for 7 d. | The active packaging system markedly increased the shelf life of Fior di Latte cheese. |
Incoronato et al., 2011
|
| 11 | UHT milk | Indirectly processed UHT milk was packaged in IntaseptTM aseptic pouches with (treatment) or without (control) oxygen-scavenging film and stored for 14 weeks at 26 +0.3 °C
.
| Oxygen content reduced (p <
0.05) by 23–28% as well as some volatile compounds contributing to flavor also reduced by 23–41% during storage. However, the consumer panel failed to detect a significant difference in odour between the treatment and control samples.
|
Perkins et al., 2007
|
|
Edible coating
|
| 1 | Gouda cheese | CNANI (water, natamycin, and nisin) and GNANI (Starch, glycerol, water, natamycin, and nisin) with final conc. of 12.5 ppm of nisin and 50 ppm of natamycin were kept for ripening at 10 ± 1 °C and at humidity 79 ± 7% controlled Chamber for 30 d. | It was observed that these coatings did not alter the physicochemical properties (pH, ash, protein, chloride, water activity, ripening index, and colour) and the development of Lactobacilli that takes place during the ripening of Gouda cheese. The GNANI applied on Gouda cheese resulted in an improved barrier against external contamination during ripening, compared to CNANI. | Berti et al., 2019 |
| 2 |
Soft cheese
Kleo
| PLA containing silicon oxide (SiOx) and other packaging material was used to pack soft cheese either in vacuum (as control) or in MAP consisting of carbon dioxide CO2 (E 290)-30% and nitrogen N2 (E 941)-70%. The samples were stored at the temperature of 4.0±0.5 °C up to 32 d.
| The shelf life was extended to 32 days, and good outside appearance and lactic acid aroma was observed. | Muizniece-Brasava et al., 2011 |
| 3 | Ricotta cheese | Cheese samples were dipped into a coating solution (0.8% w/v) of chitosan and 2.4% w/v lyophilized milk whey (11% WP) for 30 sec and packed under MAP (40% CO2/60% N2 mixture). Both samples; control and treated, werestored at 4+ 0.5 °C for 30 d. | Findings suggest a potential utility of chitosan/whey protein coatings to extend fresh dairy product shelf-life based on physico-chemical and microbial parameters. | DiPierro et al., 2011 |
| 4 | Cheese | Galactomannan (0.5% (w/v) and nisin at 50 IU/ g used to coat L. monocytogens added dried cheese samples followed by storage at 4+ 2 °C for 28 days in a cooled chamber at 75% relative humidity (RH).
| Results showed that the cheese coated with nisin-added galactomannan film presented the best results in terms of microbial growth delay (p < 0.05). | Martins et al., 2010 |
| 5 | Cheese | Chitosan, galactomannan from Gleditsia triacanthos, and agar from Glacilaria birdiae were tested, at 0.5% and 1.5% (w/v) conc.
| The uncoated cheese had an extensive mold growth at the surface when compared with the coated cheese. | Cerqueira et al., 2009 |
|
Modified atmospheric packaging (MAP)
|
| 1 | ‘‘Anthotryros’’ (whey) cheese | MAP mixtures were 30%/70 % CO2/N2 (M1) or 70%/30% CO2/N2 (M2), while VP was taken as the control sample. The samples were stored at 4 or 12 °C for 37 or 17 days, respectively.
| Both MAP conditions extended the shelf-life of fresh Anthotyros cheese stored at 4 °C by ca. 10 days (M1) or 20 days (M2) compared with VP and by ca. 2 days (M1) and 4 days (M2) at 12 °C, with cheese maintaining good sensory characteristics. | Vieira et al., 2019 |
| 2 | Yoghurt | Fermentation was done under HP (0.1, 10, 20, 30, and 40 MPa at 43 °C) followed by storage at 4 °C for 23 d. | Fermentation at higher pressure affectsthe LAB count, syneresis, and firmness. The pH remains unaffected throughout the storage. | Vieira et al., 2019 |
| 3 | Channa jalebi | Jalebi was soaked in sugar syrup containing potassium sorbate 800 ppm and packed under MAP (100% CO2, 100% N2, and 50-50% CO2and N2). The samples were stored at 28±2 °C.
| A combination of CO2 and N2was found more suitable for packaging. The shelf life of the product was 40 days.
| Geetha et al., 2017 |
| 4 |
Fresh mozzarella cheese
| Different proportions of CO2 and N2 were used to pack the samples. All samples were stored at 4 °C for 6 weeks.
| Atm 3 (packaged in 40%CO2/60% N2), exhibited the best sensory characteristics of the investigated cheese samples during the storage period. It also allowed a shelf-life extension of 46 days at 4 °C compared to vacuum.
| Felfoul et al., 2017 |
| 5 |
Soft Surface mould ripened
Cheese
| MAP-A (0% O2, 27 + 6% CO2) and MAP-B (2 + 1% O2, 19 + 2% CO2) was studied at 12 °C for 14 days.
| The shelf-life of surface mould ripened cheese with low levels of O2 (1-3%) and relatively high levels of CO2 (17-21%) extended up to 17 days.
| Rodriguez-Aguilera et al., 2011 |
| 6 | Fresh Stracciatella cheese | Combination of CO2:N2:O2 50:50:0 (M1), 95:5:0 (M2), 75:25:0 (M3), and 30:65:5 (M4) vol/vol, for packaging of cheese was used and then stored at 8 °C for 8 d.
| M1 and M2, delayed the microbial growth of spoilage bacteria, without affecting the dairy microflora, and prolonged the sensorial acceptability limit. |
Gammariello et al., 2009
|
| 7 | Whey cheese; Lor cheeses | The productwas exposed to different MAP conditions and kept in the refrigerator at 4°C for 45 days. | Sensory evaluation (odour and taste) results showed that Lor cheese packaged under modified atmosphere packaging (60% CO2⁄ 40% N2 and 70% CO2⁄ 30% N2) retained good characteristics for 45 days of storage compared to other samples.
| Temiz et al., 2009 |
| 8 |
Ricotta
| Three gas mixtures were used: 50:50 (CO2:N2), (MAP50), 70:30 (CO2:N2) (MAP70), and 95:5 (CO2:N2) (MAP95), the control sample was packed under a normal atmosphere. All samples were stored at 4 °C for 8.
|
The longest shelf life was obtained with MAP containing 95% carbon dioxide compared to the control.
|
Del Nobile et al., 2009
|
Modified atmosphere packaging
Solubilization of gas into the water phase is the basis of surrounding food with a gas mixture. It deals with the modification/alteration of the gaseous environment during the packaging of food through the removal and replacement of gas or a mixture of gases. The major gases used are CO2, N2, and O2(Ščetar et al., 2019). The N2 has no taste, acts as an inert gas, and prevents package collapse. The O2 is used almost negligible due to its negative role during the storage of non-respiring food and hence, replaced with CO2. O2 is combined with CO2 and N2 only in case of respiring food products. The permeability of packaging material towards O2, CO2, and water is essential in determining the effective atmosphere inside the package. The atmosphere is modified either passively or actively in foods(Vieira et al., 2019). In the case of former, the rate of atmosphere modification is slow and happens due to interaction between food (respiration) and gases present in the surrounding environment. The phenomenon is to be regulated by packaging material. On the other side, active modification is faster and accomplished by creating a vacuum and flushing the desired mixture of gases in the package. However, it is more expensive than the passive method. MAP has been found effective against yeast and mould growth and subsequently production of aflatoxin in varieties of cheese (Jalilzadeh et al., 2015). Other gases like carbon monoxide, helium, neon, argon, ethanol vapour, sulfur dioxide, nitrous oxide, etc. can be applied at the commercial level on a restricted basis to improve the shelf life of various foods. MAP gas leak indicator additionally confers the integrity of the package during transportation and storage. The ultraviolet-activated visual oxygen indicators have been most popularized. These indicators are composed of an electron donor, semiconductor, and redox dye. The result is indicated in the form of a change in colour. It prevents or slowsdown the microbial or chemical deterioration of food. The dairy products packed (cheese, jalebi, yoghurt) under MAP showed longer shelf life (Felfoul et al., 2017; Geetha et al., 2017).
Active packaging
The condition of packaged food is changed to extend the shelf life by the addition of active component into the packaging material. It involves various componentsthat act as active ingredients,i.e. O2 scavengers, CO2 emitting/absorbing, Ethanol emitters/scavengers, Anti-oxidants releasing, etc.(Ščetar et al., 2019) that react continuously with the inside environment of food. In addition to its function asactive packaging, smart/intelligent packaging, known as non-traditional packaging, provides information about the quality of food during transportation and storage through a device placed internally or externally on the package. Further, different types of methodology can be used in active packaging technology, e.g. releasing agents, absorbers (O2, CO2,moisture, taint), etc. iron-based scavengers under the trade name “Ageless” are produced by the leading Mitsubishi Gas Chemical Company, in Japan (Ganguly et al., 2017).
Generally, desiccants containing moisture absorbers and ethanol vapour generating material are used as active packaging for cheese. CO2 absorbents are based on either physical (active carbon powder or zeolite) or chemical (calcium hydroxide/magnesium hydroxide) composite. Moisture (Drip) absorbent pad includes super absorbent polymers carboxymethyl cellulose, modified starches and polyacrylate salts. Ethylene absorber is an example of an absorbent that scavenges off flavour-generating compounds. Aroma-Can® provides orange or lemon flavour in the food product (Haghighi-Maneshet al., 2017). Potassium permanganate, being a catalyst, oxidizes ethylene to water and CO2.The combinationof charcoal and palladium helps in delaying the softening rate of fruits and vegetables by preventing ethylene accumulation. Sometimes the antimicrobial agents viz. organic acids, bacteriocins, phenolic compounds, plant extracts, etc. are embedded within the packaging material which is designed to be released slowly and to function at the surface of a food product. The incorporation of essential oils, nano-composites of Ag, Zinc oxide, and O2 scavengers into packaging material markedly increase the shelf life of cheese and milk (Singh et al., 2018; Divsalar et al., 2018; Yang et al., 2018).
Edible coating
Biodegradable or edible packaging/coating of food opensup a new avenue for the industry. It is a thin layer made from edible material, that functionssimilarly to the conventional packaging material with aided benefits, i.e. carrier for active compounds, and potentially actsas a barrier for flavour compounds, water vapour, and gases. Based on the internal structural molecule, edible films are classified into three types; a) composites, a mixture of different hydrocolloids and lipids with each other, b) hydrocolloids, prepared from protein and polysaccharides and c) lipids. However, the mechanical strength is the main drawback of edible coating, and therefore secondary non-edible packaging is still required for proper and hygienic handling of food. Chitosan and galactomannan are mainly used for cheese packaging from the polysaccharide group. The protein-based coating material attracts the interest of industry due to its strong mechanical and barrier properties (Ščetar et al., 2019). Examples are collagen, fish proteins, wheat gluten, casein, ovalbumin, soy protein isolate, whey protein isolate, etc. Amongst all, milk proteins are found most suitable due to their nutritional value, emulsifying properties, solubility in water, and industrial surplus. The plasticizers/surfactants are usually added. The neutralor non-reacting/interfering nature of coating material with the food is an important characteristic. Various compounds such as cinnamaldehyde, linalool, carvacrol, thymol, lauramide arginineethyl ester (LAE), cocoa extract, natamycin, nisin have been incorporated into different coating/film made up of chitosan, galactomannan, polylactic acid, whey protein and have extended the shelf life of various types of cheeses and other food products (Muizniece-Brasava et al., 2011; Ungure et al., 2012; Berti et al., 2019).
Table 5. Advantages and disadvantages of the different shelf life enhancement technologies
|
Sr. No.
|
Method/Technology
|
Advantages
|
Disadvantages
|
|---|
| 1 | Thermal treatment |
Well proven in the dairy industry.
|
Destruction of heat-sensitive nutrients.
Fouling rate of equipment is high.
Supply chain must be operated under refrigeration temp.
|
| 2 | Microwave |
|
Non-uniform temperature distribution. Not suitable for solid and semi-solid food. Surface heating more rapidly than the inner part.
|
| 3 | HPP |
Rapid and uniform pressure distribution. Does not produce any significant physico-chemical changes. Retains flavour, colour,and nutritional value. Extends shelf life up to 2-3 fold.
|
|
| 4 | PEF |
Less treatment time and temperature. Retains nutritional quality. Used to pasteurize the milk.
|
High capital cost. Spores are found resistant. May not be suitable for solid foods. Air bubble interferes with the process.
|
| 5 | Cold Plasma |
Microbial inactivation at low temp. Negligible impact on the food matrix. Applied to both solid and liquid food. Energy efficient process.
|
Control of chemistry of gas plasma reactions. Oxidation in high-fat products. Difficult with bulky and irregularly shaped food. Penetration power is lower.
|
| 6 | Ohmic Heating (OH) |
Rapid and uniform heating. Reduced fouling of equipment. Low maintenance cost. Environmentally friendly process.
|
Lack of generalized information. Required adjustment according to the conductivity of the product. Difficult to monitor and control.
|
| 7 | Ultrasonication |
|
|
| 8 | Irradiation |
Used for both continuous and batch operation. Does not produce any chemical residue. Low maintenance cost. Environmentally friendly process.
|
Oxidation of protein (removal of H+ from amino acids).
Certain vitamins get affected. Opaque fluid and solid content reduce effectiveness. Human contact results incancer.
|
| 9 | MAP |
|
Increase in processing cost. Gas combination optimization. Collapse pack due to CO2.
Favours the growth of anaerobes.
|
| 10 | Edible coating |
Limits migration of aroma, flavour, and moisture. Gas barrier. Protects food. Controlled migration of additives. Zero wastage.
|
|
6. Conclusion and future prospective
Milk, yoghurt, cheese, curd or dahi, and other milk products have been consumed for thousands of years and are considered an important part of the human diet to date. The extension of shelf life is meant to preserve the nutritional quality of milk. Apart from preservation, the traditional methods of processing milk and milk products severely affect the nutrition contents, especially vitamins as well as taste, flavour, and colour. Industries are continuously engaged insearching for innovative approaches to satisfy the hunger of consumers for minimally processed and healthy food. Thus, the industry has focused on three major areas; non-thermal techniques, additives, and packaging,all of which are being continually researched. Many of them, including UHT, HPP, PEF, irradiation, and the use of biopreservatives are successfully adopted at the industry level to increase the shelf life of various types of milkand milk products. Microwave-assisted novel techniques like PEF, HHP, and Ultrasoncation have already been studied for improving the quality of food and processing efficiency producing promising results. Similarly, the combination of biopreservatives such as lysozyme bacteriocins, or EO with HHP or PEF showed tremendously encouraging results due to their synergistic effects.
The use of biopreservatives will become a great tool when it comes to prolonging shelf life in the upcoming time. However, each process has its benefits and limitations (discussed in Table 5) and therefore needs to be optimized according to the product profile. Even though cold plasma has poor penetration power such combinations can improve its effectiveness. Further, in-depth understanding would definitely help to change the mindset of consumers to facilitate the acceptance of new technologies in the future.
Contribution of the authors
N.P.S. has conceptualized, interpreted, corrected, and technically improved final version of the manuscript; A.R.P. and V.K. have provided technical suggestions, corrected it, and made it scientifically acceptable for the final version; P.P.S. has read and approved permissions for the finalization and submission of the manuscript. All the authors approved the submission of this manuscript.
Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.






